Bioactive polymeric scaffolds for tissue engineering
- PMID: 28653043
- PMCID: PMC5482547
- DOI: 10.1016/j.bioactmat.2016.11.001
Bioactive polymeric scaffolds for tissue engineering
Abstract
A variety of engineered scaffolds have been created for tissue engineering using polymers, ceramics and their composites. Biomimicry has been adopted for majority of the three-dimensional (3D) scaffold design both in terms of physicochemical properties, as well as bioactivity for superior tissue regeneration. Scaffolds fabricated via salt leaching, particle sintering, hydrogels and lithography have been successful in promoting cell growth in vitro and tissue regeneration in vivo. Scaffold systems derived from decellularization of whole organs or tissues has been popular due to their assured biocompatibility and bioactivity. Traditional scaffold fabrication techniques often failed to create intricate structures with greater resolution, not reproducible and involved multiple steps. The 3D printing technology overcome several limitations of the traditional techniques and made it easier to adopt several thermoplastics and hydrogels to create micro-nanostructured scaffolds and devices for tissue engineering and drug delivery. This review highlights scaffold fabrication methodologies with a focus on optimizing scaffold performance through the matrix pores, bioactivity and degradation rate to enable tissue regeneration. Review highlights few examples of bioactive scaffold mediated nerve, muscle, tendon/ligament and bone regeneration. Regardless of the efforts required for optimization, a shift in 3D scaffold uses from the laboratory into everyday life is expected in the near future as some of the methods discussed in this review become more streamlined.
Keywords: Bioactive; Biodegradable; Biomaterials; Porosity; Scaffold; Tissue regeneration.
Figures






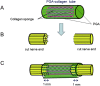

References
-
- Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Lee P., Tran K., Chang W., Fang Y.-L., Zhou G., Junka R. Bioactive polymeric scaffolds for osteochondral tissue engineering: in vitro evaluation of the effect of culture media on bone marrow stromal cells. Polym. Adv. Technol. 2015;26:1476–1485.
-
- Lee P., Tran K., Zhou G., Bedi A., Shelke N.B., Yu X. Guided differentiation of bone marrow stromal cells on co-cultured cartilage and bone scaffolds. Soft Matter. 2015;11:7648–7655. - PubMed
-
- Lee P., Tran K., Chang W., Shelke N.B., Kumbar S.G., Yu X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: cartilage regeneration. J. Biomed. Nanotechnol. 2014;10:1469–1479. - PubMed
-
- Piskin E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995;6:775–795. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
