Targeting heparin and heparan sulfate protein interactions
- PMID: 28653068
- PMCID: PMC5567684
- DOI: 10.1039/c7ob01058c
Targeting heparin and heparan sulfate protein interactions
Abstract
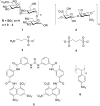
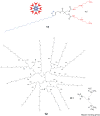
Heparin and heparan sulfate glycosaminoglycans are long, linear polysaccharides that are made up of alternating dissacharide sequences of sulfated uronic acid and amino sugars. Unlike heparin, which is only found in mast cells, heparan sulfate is ubiquitously expressed on the cell surface and in the extracellular matrix of all animal cells. These negatively-charged glycans play essential roles in important cellular functions such as cell growth, adhesion, angiogenesis, and blood coagulation. These biomolecules are also involved in pathophysiological conditions such as pathogen infection and human disease. This review discusses past and current methods for targeting these complex biomolecules as a novel therapeutic strategy to treating disorders such as cancer, neurodegenerative diseases, and infection.
Figures





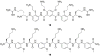

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

