Arcuate nucleus homeostatic systems reflect blood leptin concentration but not feeding behaviour during scheduled feeding on a high-fat diet in mice
- PMID: 28653356
- PMCID: PMC5601252
- DOI: 10.1111/jne.12498
Arcuate nucleus homeostatic systems reflect blood leptin concentration but not feeding behaviour during scheduled feeding on a high-fat diet in mice
Abstract
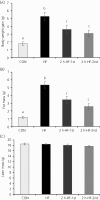
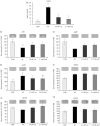
Hypothalamic homeostatic and forebrain reward-related genes were examined in the context of scheduled meal feeding without caloric restriction in C57BL/6 mice. Mice fed ad libitum but allowed access to a palatable high-fat (HF) diet for 2 hours a day rapidly adapted their feeding behaviour and consumed approximately 80% of their daily caloric intake during this 2-hour scheduled feed. Gene expression levels were examined during either the first or second hour of scheduled feeding vs 24 hours ad libitum feeding on the same HF diet. Gene expression of neuropeptide Y, agouti-related peptide, cocaine- and amphetamine-regulated transcript, pro-opiomelanocortin, long-form leptin receptor and suppressor of cytokine signalling-3 in the hypothalamic arcuate nucleus (ARC), as well as enkephalin, dynorphin, dopamine-2-receptor and dopamine-3-receptor in the nucleus accumbens (NAcc) in the forebrain, were measured by in situ hybridisation. Mice fed ad libitum on a HF diet had the highest total caloric intake, body weight gain, fat mass and serum leptin, whereas schedule-fed mice had a mild obese phenotype with intermediate total caloric intake, body weight gain, fat mass and serum leptin. The effects of feeding regime on ARC gene expression were emphasised by significant positive or negative correlations with body weight gain, fat mass and blood leptin, although they did not appear to be related to feeding behaviour in the schedule-fed groups (ie, the large, binge-type meals) and did not reveal any potential candidates for the regulation of these meals. Mechanisms underlying large meal/binge-type eating may be regulated by nonhomeostatic hedonic processes. However, assessment of opioid and dopamine receptor gene expression in the NAcc did not reveal evidence of involvement of these genes in regulating large meals. This complements our previous characterisation of ARC and NAcc genes in schedule-fed mice and rats, although it still leaves open the fundamental question about the underlying mechanisms of meal feeding.
Keywords: binge-type eating; energy balance gene expression; mice; palatable diet; scheduled feeding.
© 2017 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures


References
-
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371‐1374. - PubMed
-
- Canoy D, Buchan I. Challenges in obesity epidemiology. Obes Rev. 2007;8:1‐11. - PubMed
-
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661‐671. - PubMed
-
- Harrold JA, Dovey TM, Blundell JE, Halford JCG. CNS regulation of appetite. Neuropharmacology. 2012;63:3‐17. - PubMed
-
- Stütz AM, Staszkiewicz J, Ptitsyn A, Argyropoulos G. Circadian expression of genes regulating food intake. Obesity. 2007;15:607‐615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

