Pharmacokinetics of Monoclonal Antibodies
- PMID: 28653357
- PMCID: PMC5613179
- DOI: 10.1002/psp4.12224
Pharmacokinetics of Monoclonal Antibodies
Abstract
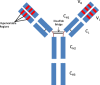
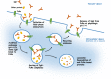
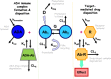
Monoclonal antibodies (mAbs) have developed in the last two decades into the backbone of pharmacotherapeutic interventions in a variety of indications, with currently more than 40 mAbs approved by the US Food and Drug Administration, and several dozens more in clinical development. This tutorial will review major drug disposition processes relevant for mAbs, and will highlight product-specific and patient-specific factors that modulate their pharmacokinetic (PK) behavior and need to be considered for successful clinical therapy.
© 2017 The Authors.
Figures


References
-
- Mould, D.R. & Meibohm, B. Drug development of therapeutic monoclonal antibodies. BioDrugs 30, 275–293 (2016). - PubMed
-
- Davis, J.D. et al Monoclonal antibodies: from structure to therapeutic application Pharmaceutical Biotechnology: Fundamentals and Applications, 4th Edition. (eds. Crommelin D.J.A., Sindelar R.D. & Meibohm B.) 143–178 (Springer, New York, NY, 2013).
-
- Dirks, N.L. & Meibohm, B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49, 633–659 (2010). - PubMed
-
- Kadir, F. , Ives, P. , Luitjens, A. & van Corven, E. Production and purification of recombinant proteins Pharmaceutical Biotechnology: Fundamentals and Applications, 4th Edition (eds. Crommelin D.J.A., Sindelar R.D. & Meibohm B.) 47–67 (Springer, New York, NY, 2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

