Syphilis testing practices in the Americas
- PMID: 28653418
- PMCID: PMC6764591
- DOI: 10.1111/tmi.12920
Syphilis testing practices in the Americas
Abstract
Objective: To present the findings of the Pan American Health Organization's 2014 survey on syphilis testing policies and practices in the Americas.
Methods: Representatives of national/regional reference and large, lower-level laboratories from 35 member states were invited to participate. A semi-structured, electronically administered questionnaire collected data on syphilis tests, algorithms, equipment/commodities, challenges faced and basic quality assurance (QA) strategies employed (i.e. daily controls, standard operating procedures, technician training, participating in external QA programmes, on-site evaluations).
Results: The 69 participating laboratories from 30 (86%) member states included 41 (59%) national/regional reference and 28 (41%) lower-level laboratories. Common syphilis tests conducted were the rapid plasma reagin (RPR) (62% of surveyed laboratories), venereal disease research laboratory (VDRL) (54%), fluorescent treponemal antibody absorption (FTA-ABS) (41%) and Treponema pallidum haemagglutination assay (TPHA) (32%). Only three facilities reported using direct detection methods, and 28 (41% overall, 32% of lower-level facilities) used rapid tests. Most laboratories (62%) used only traditional testing algorithms (non-treponemal screening and treponemal confirmatory testing); however, 12% used only a reverse sequence algorithm (treponemal test first), and 14% employed both algorithms. Another nine (12%) laboratories conducted only one type of serologic test. Although most reference (97%) and lower-level (89%) laboratories used at least one QA strategy, only 16% reported using all five basic strategies. Commonly reported challenges were stock-outs of essential reagents or commodities (46%), limited staff training (73%) and insufficient equipment (39%).
Conclusions: Many reference and clinical laboratories in the Americas face challenges in conducting appropriate syphilis testing and in ensuring quality of testing.
Keywords: Americas; Américas; Amériques; Organisation Panaméricaine de la Santé (OPS); Organización Panamericana de la Salud (OPS); Pan American Health Organization; Sífilis congénita; assurance qualité de laboratoire; congenital Syphilis; dépistage de la syphilis; eliminación de la transmisión vertical de la sífilis y el VIH; elimination of mother-to-child transmission of syphilis and HIV; garantía de calidad de laboratorio; laboratory quality assurance; prueba de sífilis; syphilis congénitale; syphilis testing; élimination de la transmission mère-enfant de la syphilis et du VIH.
© 2017 John Wiley & Sons Ltd The Pan-American Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication.
Figures
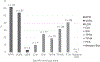
References
-
- World Health Organization (WHO). Investment Case for Eliminating Mother-to-Child Transmission of Syphilis: Promoting Better Maternal and Child Health and Stronger Health Systems. World Health Organization, 2012 http://apps.who.int/iris/bitstream/10665/75480/1/9789241504348_eng.pdf [28 October 2016].
-
- Zoni AC, Gonzalez MA, Sjogren HW. Syphilis in the most at-risk populations in Latin America and the Caribbean: a systematic review. Int J Infect Dis 2013: 17: e84–e92. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

