The mutable vaccine for mutable viruses
- PMID: 28653569
- PMCID: PMC5619096
- DOI: 10.2217/imt-2017-0030
The mutable vaccine for mutable viruses
Abstract
Mutable viruses, such as HIV, pose difficult obstacles to prevention and/or control by vaccination. Mutable viruses rapidly diversify in populations and in individuals, impeding development of effective vaccines. We devised the 'mutable vaccine' to appropriate the properties of mutable viruses that undermine conventional strategies. The vaccine consists of a DNA construct encoding viral antigen and regulatory sequences that upon delivery to B cells target the enzymatic apparatus of 'somatic hypermutation' causing the construct to mutate one million-times baseline rates and allowing production and presentation of antigen variants. We postulate the mutable vaccine might thus anticipate diversification of mutable viruses, allowing direct control or slowing of evolution. Initial work presented here should encourage consideration of this novel approach.
Keywords: B cell; HIV; antigen; escape variant; mutable virus; somatic hypermutation; vaccine; viral evolution; virus.
Conflict of interest statement
The work described in this manuscript was supported in part by grants from the National Institutes of Health (AI061100) and from the Bill and Melinda Gates Foundation (52090). M Cascalho and JL Platt hold a patent for the mutable vaccine, as cited in the manuscript and a provisional patent (62/328,131) for C3d cellular and acellular vaccines for prevention and treatment of cancer. No other conflicts of interest are pertinent to this communication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

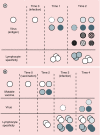


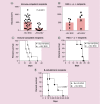
References
-
- Reitz MS, Jr, Wilson C, Naugle C, Gallo RC, Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988;54(1):57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
