Replication Study: Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia
- PMID: 28653617
- PMCID: PMC5487217
- DOI: 10.7554/eLife.25306
Replication Study: Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia
Erratum in
-
Correction: Replication Study: Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia.Elife. 2018 Jan 8;7:e34573. doi: 10.7554/eLife.34573. Elife. 2018. PMID: 29309032 Free PMC article. No abstract available.
Abstract
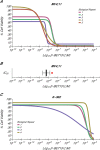
In 2015, as part of the Reproducibility Project: Cancer Biology, we published a Registered Report (Fung et al., 2015), that described how we intended to replicate selected experiments from the paper "Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia" (Dawson et al., 2011). Here, we report the results of those experiments. We found treatment of MLL-fusion leukaemia cells (MV4;11 cell line) with the BET bromodomain inhibitor I-BET151 resulted in selective growth inhibition, whereas treatment of leukaemia cells harboring a different oncogenic driver (K-562 cell line) did not result in selective growth inhibition; this is similar to the findings reported in the original study (Figure 2A and Supplementary Figure 11A,B; Dawson et al., 2011). Further, I-BET151 resulted in a statistically significant decrease in BCL2 expression in MV4;11 cells, but not in K-562 cells; again this is similar to the findings reported in the original study (Figure 3D; Dawson et al., 2011). We did not find a statistically significant difference in survival when testing I-BET151 efficacy in a disseminated xenograft MLL mouse model, whereas the original study reported increased survival in I-BET151 treated mice compared to vehicle control (Figure 4B,D; Dawson et al., 2011). Differences between the original study and this replication attempt, such as different conditioning regimens and I-BET151 doses, are factors that might have influenced the outcome. We also found I-BET151 treatment resulted in a lower median disease burden compared to vehicle control in all tissues analyzed, similar to the example reported in the original study (Supplementary Figure 16A; Dawson et al., 2011). Finally, we report meta-analyses for each result.
Keywords: Reproducibility Project: Cancer Biology; bromodomain inhibitor; cancer biology; human; leukemia; metascience; mouse; replication; reproducibility.
Conflict of interest statement
XS: Stem Cell and Xenograft Core, University of Pennsylvania, Perelman School of Medicine is a Science Exchange associated lab.
JJF: ProNovus Bioscience, LLC is a Science Exchange associated lab.
AK: ProNovus Bioscience, LLC is a Science Exchange associated lab.
GD-D: Stem Cell and Xenograft Core, University of Pennsylvania, Perelman School of Medicine is a Science Exchange associated lab.
RP:CB: EI, NP: Employed by and hold shares in Science Exchange Inc. The other authors declare that no competing interests exist.
Figures










References
-
- Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, Morschhauser F, Karlin L, Broussais F, Rezai K, Herait P, Kahatt C, Lokiec F, Salles G, Facon T, Palumbo A, Cunningham D, Zucca E, Thieblemont C. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. The Lancet Haematology. 2016;3:e196–e204. doi: 10.1016/S2352-3026(16)00021-1. - DOI - PubMed
-
- Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, Plymate SR, Navone NM, Wang S, Feng FY, Chinnaiyan AM. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of Prostate Cancer. Molecular Cancer Research. 2016;14:324–331. doi: 10.1158/1541-7786.MCR-15-0472. - DOI - PMC - PubMed
-
- Chaidos A, Caputo V, Karadimitris A. Inhibition of bromodomain and extra-terminal proteins (BET) as a potential therapeutic approach in haematological malignancies: emerging preclinical and clinical evidence. Therapeutic Advances in Hematology. 2015;6:128–141. doi: 10.1177/2040620715576662. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

