Exploitation of SPR to Investigate the Importance of Glycan Chains in the Interaction between Lactoferrin and Bacteria
- PMID: 28653977
- PMCID: PMC5539864
- DOI: 10.3390/s17071515
Exploitation of SPR to Investigate the Importance of Glycan Chains in the Interaction between Lactoferrin and Bacteria
Abstract
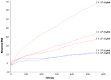
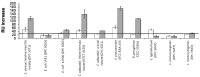
Bovine lactoferrin (LF) has been shown to prevent adhesion to and invasion of mammalian cell lines by pathogenic bacteria, with evidence for direct bacterial binding by the milk glycoprotein. However, the glycosylation pattern of LF changes over the lactation cycle. In this study, we aim to investigate the effect that this variation has on the milk glycoprotein's ability to interact with pathogens. Surface plasmon resonance technology was employed to compare the binding of LF from colostrum (early lactation) and mature milk (late lactation) to a panel of pathogenic bacteria (Staphylococcus aureus, Escherichia coli, Cronobacter sakazakii, Streptococcus pneumoniae, Pseudomonas aeruginosa, Listeria monocytogenes and Salmonella typhimurium). Novel interactions with LF were identified for C. sakazakii, S. pneumoniae and P. aeruginosa with the highest binding ability observed for mature milk LF in all cases, with the exception of S. typhimurium. The difference in bacterial binding observed may be as a result of the varying glycosylation profiles. This work demonstrates the potential of LF as a functional food ingredient to prevent bacterial infection.
Keywords: bacterial binding; glycosylation; lactation; lactoferrin; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kumar J., Weber W., Munchau S., Yadav S., Singh S.B., Saravanan K., Paramasivam M., Sharma S., Kaur P., Bhushan A., et al. Crystal structure of human seminal diferric lactoferrin at 3.4 angstrom resolution. Indian J. Biochem. Biophys. 2003;40:14–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

