Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice
- PMID: 28654087
- PMCID: PMC5490268
- DOI: 10.1038/ncomms15904
Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice
Abstract
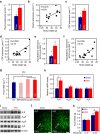
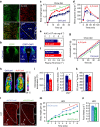
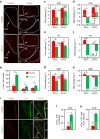
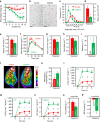
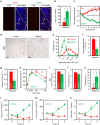
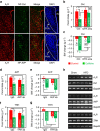
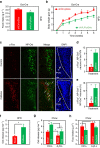
Caffeine, an antagonist of the adenosine receptor A1R, is used as a dietary supplement to reduce body weight, although the underlying mechanism is unclear. Here, we report that adenosine level in the cerebrospinal fluid, and hypothalamic expression of A1R, are increased in the diet-induced obesity (DIO) mouse. We find that mice with overexpression of A1R in the neurons of paraventricular nucleus (PVN) of the hypothalamus are hyperphagic, have glucose intolerance and high body weight. Central or peripheral administration of caffeine reduces the body weight of DIO mice by the suppression of appetite and increasing of energy expenditure. We also show that caffeine excites oxytocin expressing neurons, and blockade of the action of oxytocin significantly attenuates the effect of caffeine on energy balance. These data suggest that caffeine inhibits A1Rs expressed on PVN oxytocin neurons to negatively regulate energy balance in DIO mice.
Figures
Similar articles
-
An obligate role of oxytocin neurons in diet induced energy expenditure.PLoS One. 2012;7(9):e45167. doi: 10.1371/journal.pone.0045167. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028821 Free PMC article.
-
Differential expression of hypothalamic CART mRNA in response to body weight change following different dietary interventions.Neurochem Int. 2008 Jun;52(8):1422-30. doi: 10.1016/j.neuint.2008.03.003. Epub 2008 Mar 30. Neurochem Int. 2008. PMID: 18455834
-
Neuregulin4 Acts on Hypothalamic ErBb4 to Excite Oxytocin Neurons and Preserve Metabolic Homeostasis.Adv Sci (Weinh). 2023 Jun;10(16):e2204824. doi: 10.1002/advs.202204824. Epub 2023 Apr 14. Adv Sci (Weinh). 2023. PMID: 37060105 Free PMC article.
-
Coming full circle: contributions of central and peripheral oxytocin actions to energy balance.Endocrinology. 2013 Feb;154(2):589-96. doi: 10.1210/en.2012-1751. Epub 2012 Dec 27. Endocrinology. 2013. PMID: 23270805 Free PMC article. Review.
-
Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link?Cells. 2022 Feb 11;11(4):623. doi: 10.3390/cells11040623. Cells. 2022. PMID: 35203274 Free PMC article. Review.
Cited by
-
Chronic Intake of Energy Drinks and Their Sugar Free Substitution Similarly Promotes Metabolic Syndrome.Nutrients. 2021 Apr 6;13(4):1202. doi: 10.3390/nu13041202. Nutrients. 2021. PMID: 33917297 Free PMC article.
-
Purinergic signaling in diabetes and metabolism.Biochem Pharmacol. 2021 May;187:114393. doi: 10.1016/j.bcp.2020.114393. Epub 2020 Dec 25. Biochem Pharmacol. 2021. PMID: 33359363 Free PMC article. Review.
-
Huangshan Maofeng Green Tea Extracts Prevent Obesity-Associated Metabolic Disorders by Maintaining Homeostasis of Gut Microbiota and Hepatic Lipid Classes in Leptin Receptor Knockout Rats.Foods. 2022 Sep 20;11(19):2939. doi: 10.3390/foods11192939. Foods. 2022. PMID: 36230016 Free PMC article.
-
Aging changes the expression of adenosine receptors, insulin-like growth factor 1 (IGF1), and hypoxia-inducible factor 1α (HIF1α) in hypothalamic astrocyte cultures.Aging Brain. 2023 Dec 23;5:100104. doi: 10.1016/j.nbas.2023.100104. eCollection 2024. Aging Brain. 2023. PMID: 38225985 Free PMC article.
-
On the Nature of the Mother-Infant Tie and Its Interaction With Freudian Drives.Front Psychol. 2020 Feb 26;11:317. doi: 10.3389/fpsyg.2020.00317. eCollection 2020. Front Psychol. 2020. PMID: 32161562 Free PMC article.
References
-
- Jia W. Obesity in China: its characteristics, diagnostic criteria, and implications. Front. Med. 9, 129–133 (2015). - PubMed
-
- Bray G. A. Medical treatment of obesity: the past, the present and the future. Best Pract. Res. Clin. Gastroenterol. 28, 665–684 (2014). - PubMed
-
- Lopez-Garcia E. et al.. Changes in caffeine intake and long-term weight change in men and women. Am. J. Clin. Nutr. 83, 674–680 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

