Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer
- PMID: 28654140
- PMCID: PMC6481896
- DOI: 10.1002/14651858.CD007047.pub2
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer
Abstract
Background: Epidermal growth factor receptor (EGFR) inhibitors prevent cell growth and have shown benefit in the treatment of metastatic colorectal cancer, whether used as single agents or in combination with chemotherapy. Clear benefit has been shown in trials of EGFR monoclonal antibodies (EGFR MAb) but not EGFR tyrosine kinase inhibitors (EGFR TKI). However, there is ongoing debate as to which patient populations gain maximum benefit from EGFR inhibition and where they should be used in the metastatic colorectal cancer treatment paradigm to maximise efficacy and minimise toxicity.
Objectives: To determine the efficacy, safety profile, and potential harms of EGFR inhibitors in the treatment of people with metastatic colorectal cancer when given alone, in combination with chemotherapy, or with other biological agents.The primary outcome of interest was progression-free survival; secondary outcomes included overall survival, tumour response rate, quality of life, and adverse events.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library, Issue 9, 2016; Ovid MEDLINE (from 1950); and Ovid Embase (from 1974) on 9 September 2016; and ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) on 14 March 2017. We also searched proceedings from the major oncology conferences ESMO, ASCO, and ASCO GI from 2012 to December 2016. We further scanned reference lists from eligible publications and contacted corresponding authors for trials for further information where needed.
Selection criteria: We included randomised controlled trials on participants with metastatic colorectal cancer comparing: 1) the combination of EGFR MAb and 'standard therapy' (whether chemotherapy or best supportive care) to standard therapy alone, 2) the combination of EGFR TKI and standard therapy to standard therapy alone, 3) the combination of EGFR inhibitor (whether MAb or TKI) and standard therapy to another EGFR inhibitor (or the same inhibitor with a different dosing regimen) and standard therapy, or 4) the combination of EGFR inhibitor (whether MAb or TKI), anti-angiogenic therapy, and standard therapy to anti-angiogenic therapy and standard therapy alone.
Data collection and analysis: We used standard methodological procedures defined by Cochrane. Summary statistics for the endpoints used hazard ratios (HR) with 95% confidence intervals (CI) for overall survival and progression-free survival, and odds ratios (OR) for response rate (RR) and toxicity. Subgroup analyses were performed by Kirsten rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma RAS viral (V-Ras) oncogene homolog (NRAS) status - firstly by status of KRAS exon 2 testing (mutant or wild type) and also by status of extended KRAS/NRAS testing (any mutation present or wild type).
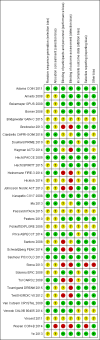
Main results: We identified 33 randomised controlled trials for analysis (15,025 participants), including trials of both EGFR MAb and EGFR TKI. Looking across studies, significant risk of bias was present, particularly with regard to the risk of selection bias (15/33 unclear risk, 1/33 high risk), performance bias (9/33 unclear risk, 9/33 high risk), and detection bias (7/33 unclear risk, 11/33 high risk).The addition of EGFR MAb to standard therapy in the KRAS exon 2 wild-type population improves progression-free survival (HR 0.70, 95% CI 0.60 to 0.82; high-quality evidence), overall survival (HR 0.88, 95% CI 0.80 to 0.98; high-quality evidence), and response rate (OR 2.41, 95% CI 1.70 to 3.41; high-quality evidence). We noted evidence of significant statistical heterogeneity in all three of these analyses (progression-free survival: I2 = 76%; overall survival: I2 = 40%; and response rate: I2 = 77%), likely due to pooling of studies investigating EGFR MAb use in different lines of therapy. Rates of overall grade 3 to 4 toxicity, diarrhoea, and rash were increased (moderate-quality evidence for all three outcomes), but there was no evidence for increased rates of neutropenia.For the extended RAS wild-type population (no mutations in KRAS or NRAS), addition of EGFR MAb improved progression-free survival (HR 0.60, 95% CI 0.48 to 0.75; moderate-quality evidence) and overall survival (HR 0.77, 95% CI 0.67 to 0.88; high-quality evidence). Response rate was also improved (OR 4.28, 95% CI 2.61 to 7.03; moderate-quality evidence). We noted significant statistical heterogeneity in the progression-free survival analysis (I2 = 61%), likely due to the pooling of studies combining EGFR MAb with chemotherapy with monotherapy studies.We observed no evidence of a statistically significant difference when EGFR MAb was compared to bevacizumab, in progression-free survival (HR 1.02, 95% CI 0.93 to 1.12; high quality evidence) or overall survival (HR 0.84, 95% CI 0.70 to 1.01; moderate-quality evidence). We noted significant statistical heterogeneity in the overall survival analysis (I2 = 51%), likely due to the pooling of first-line and second-line studies.The addition of EGFR TKI to standard therapy in molecularly unselected participants did not show benefit in limited data sets (meta-analysis not performed). The addition of EGFR MAb to bevacizumab plus chemotherapy in people with KRAS exon 2 wild-type metastatic colorectal cancer did not improve progression-free survival (HR 1.04, 95% CI 0.83 to 1.29; very low quality evidence), overall survival (HR 1.00, 95% CI 0.69 to 1.47; low-quality evidence), or response rate (OR 1.20, 95% CI 0.67 to 2.12; very low-quality evidence) but increased toxicity (OR 2.57, 95% CI 1.45 to 4.57; low-quality evidence). We noted significant between-study heterogeneity in most analyses.Scant information on quality of life was reported in the identified studies.
Authors' conclusions: The addition of EGFR MAb to either chemotherapy or best supportive care improves progression-free survival (moderate- to high-quality evidence), overall survival (high-quality evidence), and tumour response rate (moderate- to high-quality evidence), but may increase toxicity in people with KRAS exon 2 wild-type or extended RAS wild-type metastatic colorectal cancer (moderate-quality evidence). The addition of EGFR TKI to standard therapy does not improve clinical outcomes. EGFR MAb combined with bevacizumab is of no clinical value (very low-quality evidence). Future studies should focus on optimal sequencing and predictive biomarkers and collect quality of life data.
Conflict of interest statement
David Lok Hang Chan has no competing interests to declare.
Eva Segelov has received travel grant support from Roche, Merck Serono, Amgen, and Sanofi‐Aventis. She has attended advisory boards for Roche, Merck Serono, and Sanofi‐Aventis.
Rachel Wong has no competing interests to declare.
Annabel Smith has no competing interests to declare.
Rebecca A Herbertson has no competing interests to declare.
Bob Li has no competing interests to declare.
Niall Tebbutt has received speaking honoraria and travel grant support from Roche, Amgen, Merck, and Sanofi‐Aventis. He has attended advisory boards for Roche, Sanofi‐Aventis, Amgen, and Merck.
Timothy Price has received speaking honoraria or travel grant support, or both from Roche, Amgen, Pfizer, and Sanofi‐Aventis. He has attended advisory boards for Roche, Merck Australia, Amgen, and Sanofi‐Aventis, and received research funding from Sanofi‐Aventis.
Nick Pavlakis has received speaking honoraria and travel grant support from Roche, Amgen, and Sanofi‐Aventis. He has attended advisory boards for Roche, Alphapharm, Amgen, and Sanofi‐Aventis, and received research funding from Sanofi‐Aventis.
Figures






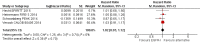
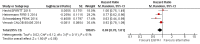

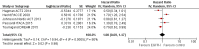
Update of
- doi: 10.1002/14651858.CD007047
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy.Cochrane Database Syst Rev. 2015 Dec 1;2015(12):CD010341. doi: 10.1002/14651858.CD010341.pub2. Cochrane Database Syst Rev. 2015. PMID: 26625332 Free PMC article.
-
Adjuvant epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for the treatment of people with resected stage I to III non-small-cell lung cancer and EGFR mutation.Cochrane Database Syst Rev. 2025 May 27;5(5):CD015140. doi: 10.1002/14651858.CD015140.pub2. Cochrane Database Syst Rev. 2025. PMID: 40421698 Review.
-
Next-generation sequencing for guiding matched targeted therapies in people with relapsed or metastatic cancer.Cochrane Database Syst Rev. 2025 Mar 24;3(3):CD014872. doi: 10.1002/14651858.CD014872.pub2. Cochrane Database Syst Rev. 2025. PMID: 40122129
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Silencing of miR490-3p by H. pylori activates DARPP-32 and induces resistance to gefitinib.Cancer Lett. 2020 Oct 28;491:87-96. doi: 10.1016/j.canlet.2020.07.014. Epub 2020 Jul 29. Cancer Lett. 2020. PMID: 32735911 Free PMC article.
-
Digestive cancers: mechanisms, therapeutics and management.Signal Transduct Target Ther. 2025 Jan 15;10(1):24. doi: 10.1038/s41392-024-02097-4. Signal Transduct Target Ther. 2025. PMID: 39809756 Free PMC article. Review.
-
Effectiveness and safety of monoclonal antibodies for metastatic colorectal cancer treatment: systematic review and meta-analysis.Ecancermedicalscience. 2015 Oct 15;9:582. doi: 10.3332/ecancer.2015.582. eCollection 2015. Ecancermedicalscience. 2015. PMID: 26557880 Free PMC article. Review.
-
Screening of Therapeutic Candidate Genes of Quercetin for Cervical Cancer and Analysis of Their Regulatory Network.Onco Targets Ther. 2021 Feb 5;14:857-866. doi: 10.2147/OTT.S287633. eCollection 2021. Onco Targets Ther. 2021. PMID: 33574679 Free PMC article.
-
Epidermal Growth Factor Receptor Inhibitor Treatment Timing does not Impact Survival in Stage 4 Colon Cancer Treatment: A Retrospective Study.Kans J Med. 2022 Aug 22;15(2):273-277. doi: 10.17161/kjm.vol15.15975. eCollection 2022. Kans J Med. 2022. PMID: 36042840 Free PMC article.
References
References to studies included in this review
Adams COIN 2011 {published data only (unpublished sought but not used)}
-
- Adams RA, Fisher D, Farragher S, Jasani B, Smith CG, James MD, et al. Use of epiregulin (EREG) and amphiregulin (AREG) gene expression to predict response to cetuximab (cet) in combination with oxaliplatin (Ox) and 5FU in the first‐line treatment of advanced colorectal cancer (aCRC) [COIN trial]. Journal of Clinical Oncology 2012;30(Suppl):Abstr 32. [10.1200/jco.2012.30.30_suppl.32 ]
-
- Adams RA, Fisher D, Farragher S, Scott A, Smith CG, James MD, et al. Epiregulin (EREG) and amphiregulin (AREG) gene expression to predict response to cetuximab therapy in combination with oxaliplatin (Ox) and 5FU in first‐line treatment of advanced colorectal cancer (aCRC) [COIN Trial]. Journal of Clinical Oncology 2012;30(Suppl):Abstr 3516.
-
- Adams RA, James MD, Smith CG, Wilson RH, Fisher D, Kenny SL, et al. Epidermal growth factor receptor (EGFR) as a predictive and prognostic marker in patients with advanced colorectal cancer (aCRC): The MRC COIN trial experience [COIN Trial]. Journal of Clinical Oncology Conference: 2011 Gastrointestinal Cancers Symposium 2011;29(Suppl 4):Abstr 359.
-
- Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, et al. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2013‐4. [:10.1016/S0140‐ 6736(11)60613‐2] - PMC - PubMed
Amado 2008 {published data only}
-
- Patterson SD, Peeters M, Siena S, Cutsem E, Humblet Y, Laethem J, et al. Comprehensive analysis of KRAS and NRAS mutations as predictive biomarkers for single agent panitumumab (pmab) response in a randomized, phase III metastatic colorectal cancer (mCRC) study (20020408). Journal of Clinical Oncology 2013;31:(Suppl; abstr 3617).
-
- Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. Journal of Clinical Oncology 2007;25(13):1658‐64. - PubMed
Bokemeyer OPUS 2009 {published data only}
-
- Bokemeyer C, Bondarenko I, Hartmann JT, Baurd F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX‐4 as first‐line treatment for metastatic colorectal cancer. Annals of Oncology 2011;22(7):1535‐46. - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first‐line treatment of metastatic colorectal cancer. Journal of Clinical Oncology 2009;27(5):663‐71. [DOI: 10.1200/JCO.2008.20.8397] - DOI - PubMed
-
- Bokemeyer C, Boondarendo I, Hartmann JT, Braud F, Schuch G, Zubel A, et al. Overall survival of patients with KRAS wild‐type tumours treated with FOLFOX4 +/‐ cetuximab as 1st‐line treatment for metastatic colorectal cancer. European Journal of Cancer 2009;7(2):346. [Abstract number: 6079]
Borner 2008 {published and unpublished data}
-
- Borner M, Koeberle D, Moos R, Saletti P, Rauch D, Hess V, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first‐line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Annals of Oncology 2008;19(7):1288‐92. [DOI: 10.1093/annonc/mdn05] - DOI - PubMed
-
- Borner M, Mingrone W, Koeberle D, Moos R, Rauch D, Saletti P, et al. The impact of cetuximab on the capecitabine plus oxaliplatin (XELOX) combination in first‐line treatment of metastatic colorectal cancer (MCC): A randomized phase II trial of the Swiss Group for Clinical Cancer Research (SAKK). Journal of Clinical Oncology 2006;24(18S):3551. - PubMed
Bridgewater GAIN‐C 2015 {published data only (unpublished sought but not used)}
-
- Bridgewater JA, Cervantes A, Markman B, Siena S, Cubillo A, Carbonero RG, et al. GAIN‐(C): Efficacy and safety analysis of imgatuzumab (GA201), a novel dual‐acting monoclonal antibody (mAb) designed to enhance antibody‐dependent cellular cytotoxicity (ADCC), in combination with FOLFIRI compared to cetuximab plus FOLFIRI in second‐line KRAS exon 2 wild type (e2WT) or with FOLFIRI alone in mutated (e2MT) metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2015;33:(Suppl 3; abstr 669).
-
- Cervantes‐Ruiperez A, Markman B, Siena S, Pericay C, Aprile G, Bridgewater JA, et al. The GAIN‐C study (BP25438): Randomized phase II trial of RG7160 (GA201) plus FOLFIRI, compared to cetuximab plus FOLFIRI or FOLFIRI alone in second‐line KRAS wild type (WT) or mutant metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2012;30:(Suppl; abstr TPS3637).
Brodowicz 2013 {published data only}
-
- Brodowicz T, Ciuleanu T, Radosavljevic D, Shacham‐Shmueli E, Vrbanec D, Plate S, et al. FOLFOX4 plus cetuximab administered weekly or every second week in the first‐line treatment of patients with KRAS wild‐type metastatic colorectal cancer: a randomized phase II CECOG study. Annals of Oncology 2013;24:1769‐77. - PubMed
-
- Brodowicz T, Vrbanec D, Kaczirek K, Ciuleanu T, Knittelfelder R, Lindner E, et al. FOLFOX4 plus cetuximab administered weekly or every two weeks in first‐line treatment of patients with KRAS andNRAS wild‐type (wt) metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2014;32(Suppl 3):abstr LBA391^.
-
- Ciuleanu T, Nikolic V, Shmueli E, Vrbanec D, Plate S, Krmpotic ZM, et al. Cetuximab weekly (q1w) versus every two weeks (q2w) plus FOLFOX4 as first‐line therapy in patients (pts) with KRAS wild‐type (wt) metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2011;29:(Suppl; abstr 3580).
Ciardiello CAPRI‐GOIM 2016 {published data only}
-
- Ciardiello F, Barone C, Cartenì G, Rachiglio AM, Montesarchio V, Tonini G, et al. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next‐generation sequencing: findings from the CAPRI‐GOIM trial. Annals of Oncology 2014;25:1756‐61. - PubMed
-
- Ciardiello F, Maiello E, Pisconti S, Giuliani F, Barone C, Rizzo M, et al. Optimal treatment strategy in KRAS wild type (wt) metastatic colorectal cancer (mCRC): Cetuximab plus FOLFIRI followed by FOLFOX4 with or without cetuximab ‐ the Capri trial from the Gruppo Oncologico Dell’Italia Meridionale (GOIM). Journal of Clinical Oncology 2013;31:Suppl; abstr e14565.
-
- Ciardiello F, Normanno N, Martinelli E, Troiani T, Cardone C, Nappi A, et al. Cetuximab beyond progression in RAS wild type (WT) metastatic colorectal cancer (mCRC): the CAPRI‐GOIM randomized phase II study of FOLFOX versus FOLFOX plus cetuximab. Annals of Oncology 2015;26 (Suppl 4):iv120‐1: Abstr LBA‐09.
-
- Ciardiello F, Normanno N, Martinelli E, Troiani T, Pisconti S, Cardone C, et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI‐GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Annals of Oncology 2016;27(6):1055‐61. - PubMed
Douillard PRIME 2010 {published data only}
-
- Douillard J, Cassidy J, Jassem J, Rivera F, Kocakova I, Rogowski W, et al. Randomized, open‐label, phase III study of panitumumab (pmab) with FOLFOX4 versus FOLFOX4 alone as first‐line treatment (tx) for metastatic colorectal cancer (mCRC): Efficacy by skin toxicity (ST). Journal of Clinical Oncology 2010;28(15 Suppl):Abstr 3528. [10.1200/jco.2010.28.15_suppl.3528 ]
-
- Douillard J, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized phase 3 study of panitumumab (PMAB) with folfox4 compared to folfox4 alone as first line treatment (TX) for metastatic colorectal cancer (MCRC): prime trial. Journal of Clinical Oncology 2010;28(31):4697‐705. [DOI: 10.1200/JCO.2009.27.4860] - DOI - PubMed
-
- Douillard J, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel ME, et al. Randomized phase 3 study of panitumumab with FOLFOX4 compared to FOLFOX4 alone as 1st‐line treatment (tx) for metastatic colorectal cancer (mCRC). Joint ECCO 15 ‐ 34th ESMO Multidisciplinary Congress; 2009 Sep 20‐24; Berlin, Germany. 2009.
-
- Douillard J, Siena S, Cassidy J, Tabernero J, Burkes RL, Barugel ME, et al. Final results from PRIME: Randomized phase III study of panitumumab (pmab) with FOLFOX4 for first line metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2011;29(Suppl):Abstr 3510. - PubMed
-
- Douillard J, Siena S, Tabernero J, Burkes RL, Barugel ME, Humblet Y, et al. Overall survival (OS) analysis from PRIME: Randomized phase III study of panitumumab (pmab) with FOLFOX4 for first‐line metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2013;31(Suppl):Abstr 3620.
Hagman ACT2 2014 {published and unpublished data}
-
- Hagman H, Frodin J, Berglund AM, Sundberg J, Vestermark LW, Albertsson M, et al. A randomized study of KRAS stratified maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first line induction treatment of metastatic colorectal cancer: the Nordic ACT2 trial. Annals of Oncology 2014;25(Suppl 4):iv167‐209, 516P. - PubMed
-
- Hagman H, Frodin JE, Berglund A, Sundberg J, Vestermark LW, Albertsson M, et al. A randomized study of KRAS‐guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first‐line induction treatment of metastatic colorectal cancer: the Nordic ACT2 trial. Annals of Oncology 2016;27(1):140‐7. - PubMed
Hecht PACCE 2009 {published data only}
-
- Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. Journal of Clinical Oncology 2008;27:672‐80. [DOI: 10.1200/JCO.2008.19.8135] - DOI - PubMed
Hecht SPIRITT 2015 {published data only}
-
- Cohn AL, Krishnan K, Hecht JR. SPIRITT: A multicenter, open‐label, randomized, phase II clinical trial evaluating safety and efficacy of FOLFIRI with either panitumumab or bevacizumab as second‐line treatment in patients with metastatic colorectal cancer (mCRC) with wild‐type KRAS tumors. Journal of Clinical Oncology 2010;28 (Suppl 15):30. [abstract no.TPS195]
-
- Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline‐Burkhardt M, et al. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second‐line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clinical Colorectal Cancer 2015;14(2):72‐80. - PubMed
-
- Hecht JR, Cohn AL. SPIRITT: A study of second‐line treatment of metastatic colorectal cancer with FOLFIRI plus panitumumab or bevacizumab. Community Oncology 2008;5(11 (Suppl B)):1‐4.
-
- Hecht JR, Cohn AL, Dakhil SR, Saleh MN, Piperdi B, Cline‐Burkhardt VJM, et al. SPIRITT (study 20060141): A randomized phase II study of FOLFIRI with either panitumumab (pmab) or bevacizumab (bev) as second ‐line treatment (tx) in patients (pts) with wild‐type (WT) KRAS metastatic colorectal cancer (mCRC) [SPIRITT]. Journal of Clinical Oncology 2013;31(4 Suppl):Abstr 454.
-
- Hecht JR, Dakhil SR, Saleh MN, Piperdi B, Cline‐Burkhardt M, Kocs DM, et al. Pooled safety results from SPIRITT: A multicenter, open‐label, randomized, phase II study of FOLFIRI with panitumumab (pmab) or bevacizumab (bev) as second‐line treatment (tx) in patients (pts) with metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2011;29(4 Suppl):Abstr 477.
Heinemann FIRE‐3 2014 {published data only}
-
- Heinemann V, Fischer von Weikersthal L, Decker T, Kiani A, Vehling‐Kaiser U, Al‐Batran S, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment of KRAS wild‐type metastatic colorectal cancer: German AIO study KRK‐0306 (FIRE‐3). Journal of Clinical Oncology 2013;31(18 Suppl):LBA3506. [DOI: 10.1200/jco.2013.31.18_suppl.lba3506] - DOI
-
- Heinemann V, Weikersthal LF, Decker T, Kiani A, Vehling‐Kaiser U, Al‐Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): a randomised, open‐label, phase 3 trial. Lancet Oncology 2014;15(10):1065‐75. [DOI: 10.1016/S1470-2045(14)70330-4] - DOI - PubMed
-
- Stintzing S, Fischer von Weikersthal L, Decker T, Vehling‐Kaiser U, Jager E, Heintges T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer ‐ subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK‐0306. Annals of Oncology 2012;23(7):1693‐9. - PubMed
-
- Stintzing S, Neumann J, Jung A, Fischer von Weikersthal L, Decker T, Vehling‐Kaiser U, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (mCRC): Analysis of patients with KRAS‐mutated tumors in the randomized German AIO study KRK‐0306. Journal of Clinical Oncology 2011;29(15 Suppl):3575. - PubMed
Hickish 2014 {published and unpublished data}
Johnsson Nordic ACT 2013 {published data only}
-
- Johnsson A, Frodin J, Berglund A, Hagman H, Sundberg J, Bergstrom D, et al. A randomized phase III trial on maintenance treatment with bevacizumab (bev) alone or in combination with erlotinib (erlo) after chemotherapy and bev in metastatic colorectal cancer (mCRC). Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011 2011;29(15 Suppl):3526. [DOI: 10.1200/jco.2011.29.15_suppl.3526] - DOI
-
- Johnsson A, Hagman H, Frodin JE, Berglund A, Keldsen N, Fernebro E, et al. A randomized phase III trial on maintenance treatment with bevacizumab alone or in combination with erlotinib after chemotherapy and bevacizumab in metastatic colorectal cancer: the Nordic ACT Trial. Annals of Oncology 2013;24(9):2335‐41. - PubMed
Karapetis CO17 2008 {published data only (unpublished sought but not used)}
-
- Asmis TR, Powell E, Karapetis CS, Jonker DJ, Tu D, Jeffery M, et al. Comorbidity, age and overall survival in cetuximab‐treated patients with advanced colorectal cancer (ACRC) ‐ results from NCIC CTG CO.17: a phase III trial of cetuximab versus best supportive care. Annals of Oncology 2011;22(1):118‐26. [DOI: 10.1093/annonc/mdq309] - DOI - PubMed
-
- Au HJ, Karapetis CS, O'Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, et al. Health‐related quality of life in patients with advanced colorectal cancer treated with cetuximab: Overall and KRAS‐specific results of the NCIC CTG and AGITG CO.17 Trial. Journal of Clinical Oncology 2009;27(11):1822‐8. [DOI: 10.1200/JCO.2008.19.6048] - DOI - PubMed
-
- Elimova E, O'Callaghan CJ, Tu D, Karapetis CS, Price TJ, Zhu L, et al. Cetuximab (CET)‐related hypersensitivity reactions (HSRs): An analysis of timing, demographics, and outcomes from the AGITG/NCIC CTG CO.17 trial. Journal of Clinical Oncology 2011;29(15 Suppl):3624. [DOI: 10.1200/jco.2011.29.15_suppl.3624] - DOI
-
- Jonker DJ, Karapetis CS, O'Callaghan CJ, Marginean C, Zalcberg JR, Simes J, et al. BRAF, PIK3CA, and PTEN status and benefit from cetuximab (CET) in the treatment of advanced colorectal cancer (CRC): Results from NCIC CTG/AGITG CO.17. Journal of Clinical Oncology 2012;30(15 Suppl):3515. - PubMed
-
- Jonker DJ, Karapetis C, Harbison C, O'Callaghan CJ, Tu D, Simes RJ, et al. High epiregulin (EREG) gene expression plus K‐ras wild‐type (WT) status as predictors of cetuximab benefit in the treatment of advanced colorectal cancer (ACRC): Results from NCIC CTG CO.17‐A phase III trial of cetuximab versus best supportive care (BSC). Journal of Clinical Oncology 2009;27(15 Suppl):4016.
Ma 2013 {published data only (unpublished sought but not used)}
-
- Ma BB, Chan SL, Ho WM, Lau W, Mo F, Hui EP, et al. Intermittent versus continuous erlotinib with concomitant modified "XELOX" (q3W) in first‐line treatment of metastatic colorectal cancer: correlation with serum amphiregulin and transforming growth factor alpha. Cancer 2013;119(23):4145‐53. - PubMed
Passardi ITACA 2015 {published data only}
-
- Fabbri F, Passardi A, Ravaioli A, Cavanna L, Luppi G, Mucciarini C, et al. Sequential treatment strategy for metastatic colorectal cancer: A phase III study of chemotherapy (CT) with or without bevacizumab (Bev) as first‐line therapy followed by two phase III studies of CT alone or CT plus bev with or without cetuximab (Cetux) as second‐line therapy (ITACa Study IRST 153 01). Journal of Clinical Oncology Conference, ASCO Annual Meeting 2011;29(15 Suppl):Abstr e14161.
-
- Passardi A, Scarpi E, Fontana A, Cavanna L, Ruscelli S, Turci D, et al. Impact of second‐line cetuximab‐containing therapy in patients with KRAS wild type metastatic colorectal cancer: results from ITACa trial. Annals of Oncology 2015;26 (Suppl 4):iv83‐4: Abstr P‐283.
Peeters 2010 {published data only (unpublished sought but not used)}
-
- Andre T, Peeters M, Price T, Hotko Y, Cervantes A, Ducreux M, et al. Panitumumab with FOLFIRI vs FOLFIRI alone: A randomised phase 3 study for the second line treatment of patients (PTS) with metastatic colorectal cancer (mCRC). Annals of Oncology 2010;21(Suppl 1):I13. - PubMed
-
- Oliner K, Peeters M, Siena S, Cutsem E, Huang J, Humblet Y, et al. Evaluation of gene mutations beyond KRAS as predictive biomarkers of response to panitumumab in a randomized, phase III monotherapy study of metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2011;29(15 Suppl):3530.
-
- Patterson SD, Peeters M, Siena S, Cutsem E, Humblet Y, Laethem J, et al. Comprehensive analysis of KRAS and NRAS mutations as predictive biomarkers for single agent panitumumab (pmab) response in a randomized, phase III metastatic colorectal cancer (mCRC) study (20020408). Journal of Clinical Oncology 2013;31(15 Suppl):3617.
-
- Peeters M, Cervantes‐Ruiperez A, Strickland A, Ciuleanu T, Mainwaring PN, Tzekova VI, et al. Randomized phase III study of panitumumab (pmab) with FOLFIRI versus FOLFIRI alone as second‐line treatment (tx) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis by tumor epidermal growth factor receptor (EGFR) staining. Journal of Clinical Oncology 2010;28:15s.
Polikoff EXPLORE 2005 {published data only (unpublished sought but not used)}
-
- Polikoff J, Mitchell EP, Badarinath S, Graham CD, Jennis A, Chen TT, et al. Cetuximab plus FOLFOX for colorectal cancer (EXPLORE): Preliminary efficacy analysis of a randomized phase III trial. Journal of Clinical Oncology 2005;23 (16S):3574.
Price ASPECCT 2014 {published data only}
-
- Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy‐refractory wild‐type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open‐label, non‐inferiority phase 3 study. Lancet Oncology 2014;15(6):569‐79. - PubMed
Santoro 2008 {published data only (unpublished sought but not used)}
-
- Santoro A, Comandone A, Rimassa L, Granetti C, Lorusso V, Oliva C, et al. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer.. Annals of Oncology 2008;19(11):1888‐93. - PubMed
Schwartzberg PEAK 2014 {published data only}
-
- Karthaus M, Schwartzberg L, Rivera F, Fasola G, Canon JL, Yu H, et al. Updated overall survival (OS) analysis of novel predictive KRAS/NRAS mutations beyond KRAS exon 2 in PEAK: A 1st‐line phase 2 study of FOLFOX6 plus panitumumab (pmab) or bevacizumab (bev) in metastatic colorectal cancer (mCRC). European Journal of Cancer 2013;49 (Suppl 2):Abstr 2262.
-
- Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild‐type KRAS exon 2 metastatic colorectal cancer. Journal of Clinical Oncology 2014;32(21):2240‐7. - PubMed
-
- Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Yu H, et al. Analysis of KRAS/NRAS mutations in PEAK: A randomized phase II study of FOLFOX6 plus panitumumab (pmab) or bevacizumab (bev) as first‐line treatment (tx) for wild‐type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2013;31:Suppl; abstr 3631.
-
- Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Yu H, et al. PEAK (study 20070509): A randomized phase II study of mFOLFOX6 with either panitumumab (pmab) or bevacizumab (bev) as first‐line treatment (tx) in patients (pts) with unresectable wild‑type (WT) KRAS metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2013;31:Suppl 4; abstr 446.
Seymour PICCOLO 2013 {published data only (unpublished sought but not used)}
-
- Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild‐type, fluorouracil‐resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncology 2013;14:749‐59. [10.1016/ S1470‐2045(13)70163‐3] - PMC - PubMed
-
- Seymour MT, Brown SR, Richman S, Middleton GW, Maughan T, Olivier C, et al. Addition of panitumumab to irinotecan: Results of PICCOLO, a randomized controlled trial in advanced colorectal cancer (aCRC). Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29(15 Suppl):3523.
-
- Seymour MT, Brown SR, Richman S, Middleton GW, Maughan TS, Maisey N, et al. Panitumumab in combination with irinotecan for chemoresistant advanced colorectal cancer: Results of PICCOLO, a large randomised trial with prospective molecular stratification. European Journal of Cancer 2011;47(Suppl 3):S393.
Siena 2013 {published data only}
Sobrero EPIC 2008 {published data only (unpublished sought but not used)}
-
- Abubakr Y, Eng C, Wong L, Pautret V, Scheithauer W, Maurel J, et al. Cetuximab plus irinotecan for metastatic colorectal cancer (mCRC): Safety analysis of 800 patients in a randomized phase III trial (EPIC). Journal of Clinical Oncology 2006;24:3556.
-
- Langer C, Kopit J, Awad M, Williams K, Teegarden P, Xu L, et al. Analysis of K‐RAS mutations in patients with metastatic colorectal cancer receiving cetuximab in combination with irinotecan: results from the EPIC trial. Annals of Oncology 2009;19((Suppl 8)):133 Abstract No: 385P.
-
- Sobrero A, Scheithauer W, Maurel J, Mineur L, Fehrenbacher L, Kisker O, et al. Cetuximab plus irinotecan for metastatic colorectal cancer (mCRC): safety analysis of the first 400 patients in a randomized phase III trial (EPIC). Annual Meeting Proceedings of the American Society of Clinical Oncology, Abstract 2005;23(16 Suppl):266.
-
- Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. Journal of Clinical Oncology 2008;26(14):2311‐9. [DOI: 10.1200/JCO.2007.13.1193] - DOI - PubMed
-
- Yang D, Bohanes P, Zhang W, Harbison C, Trifan OC, Ning Y, et al. Association of HER2 and IGF1 germline polymorphisms with outcome in metastatic colorectal cancer (mCRC) patients (pts) treated with second‐line irinotecan (IR) with or without cetuximab (CB): The EPIC experience. Journal of Clinical Oncology 2012;30(2 Suppl):3615.
Tol CAIRO2 2008 {published data only}
-
- Tol J, Dijkstra JR, Klomp M, Teerenstra S, Dommerholt M, Vink‐Börger ME, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. European Journal of Cancer 2010;46(11):1997‐2009. - PubMed
-
- Tol J, Dijkstra JR, Vink‐Borger ME, Koopman M, Vincent AD, Krieken JHJM, et al. BRAF mutation is associated with a decreased outcome in patients (pts) with advanced colorectal cancer (ACC) treated with chemotherapy and bevacizumab with or without cetuximab. European Journal of Cancer 2009;7(2):321.
-
- Tol J, Koopman M, Antonini NF, Sinnige H, Valster FAA, Braun JJ, et al. Randomized phase III study of capecitabine, oxaliplatin and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). Annals of Oncology 2009;19(Suppl 8):128. - PubMed
-
- Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. New England Journal of Medicine 2009;360(6):563‐72. - PubMed
-
- Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first‐line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Annals of Oncology 2008;19(4):734‐8. - PubMed
Tournigand DREAM 2015 {published data only}
-
- Tournigand C, Chibaudel B, Samson B, Scheithauer W, Vernerey D, Mesange P, et al. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): a randomised, open‐label, phase 3 trial. Lancet Oncology 2015;16:1493‐505. - PubMed
-
- Tournigand C, Samson B, Scheithauer W, Lledo G, Viret F, Andre T, et al. Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first‐line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial.. Journal of Clinical Oncology 2012;30:Suppl; abstr LBA3500.
Tveit NORDIC VII 2012 {published data only (unpublished sought but not used)}
-
- Tveit K, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Randomized phase III study of 5‐fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first‐line treatment of metastatic colorectal cancer: The NORDIC VII study (NCT00145314), by the Nordic Colorectal Cancer Biomodulation Group. Journal of Clinical Oncology 2011;29(4 Suppl):365.
-
- Tveit K, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Randomized phase III study of 5‐fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first‐line treatment of metastatic colorectal cancer: The Nordic VII study (NCT00145314), by the Nordic Colorectal Cancer Biomodulation Group. Annals of Oncology 2010;21(Suppl 8):viii9.
-
- Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first‐line treatment of metastatic colorectal cancer: the NORDIC‐VII study. Journal of Clinical Oncology 2012;30(15):1755‐62. - PubMed
Van Cutsem CRYSTAL 2009 {published data only (unpublished sought but not used)}
-
- Folprecht G, Nowacki M, Lang I, Cascinu S, Shchepotin I, Maurel J, et al. Cetuximab plus FOLFIRI first line in patients (pts) with metastatic colorectal cancer (mCRC): A quality‐of‐life (QoL) analysis of the CRYSTAL trial. Journal of Clinical Oncology 2009;27(15 Suppl):4076.
-
- Folprecht G, Nowacki M, Lang I, Cascinu S, Shchepotin I, Maurel J, et al. Cetuximab plus FOLFIRI first line in patients (pts) with metastatic colorectal cancer (mCRC): A quality‐of‐life (QoL) analysis of the CRYSTAL trial. Journal of Clinical Oncology 2009;27(15S Part I):187 (abstract no. 4076).
-
- Kohne C, Stroiakovski D, Chang‐chien C, Lim R, Pinter T, Bodoky G, et al. Predictive biomarkers to improve treatment of metastatic colorectal cancer (mCRC): Outcomes with cetuximab plus FOLFIRI in the CRYSTAL trial. Journal of Clinical Oncology 2009;27(15 Suppl):4068.
-
- Lang I, Aleknaviciene B, Zolotukhin S, Komissarenko V, Garcia‐Alfonso P, Jurga L, et al. Impact on quality of life of cetuximab plus FOLFIRI in the first‐line treatment of patients with metastatic colorectal cancer (MCRC): results from the CRYSTAL trial. Annals of Oncology 2009;20:23‐4 (suppl 7; abstr PD‐0024).
-
- Lang I, Kohne CH, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, et al. Cetuximab plus FOLFIRI in 1st‐line treatment of metastatic colorectal cancer: Quality of life (QoL) analysis of patients (pts) with KRAS wild‐type (wt) tumours in the CRYSTAL trial. European Journal of Cancer, Supplement Conference: Joint ECCO 15 ‐ 34th ESMO Multidisciplinary Congress; 2009 Sept 20‐24; Berlin, Germany 2009;7(2):345.
Venook CALGB 80405 2014 {published data only}
-
- Anonymous. CALGB/SWOG C80405: A phase III trial of FOLFIRI or FOLFOX with bevacizumab or cetuximab or both for untreated metastatic adenocarcinoma of the colon or rectum. Clinical Advances in Hematology & Oncology 2006;4(6):452‐3. - PubMed
-
- Naughton MJ, Schrag D, Venook AP, Niedzwiecki D, Anderson RT, Lenz H‐J, et al. Quality of life (QOL) and toxicity among patients in CALGB 80405. Journal of Clinical Oncology 2013;31:Suppl 4; abstr 3611.
-
- Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Mahoney MR, O'Neil BH, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5‐FU/leucovorin (FOLFIRI) or oxaliplatin/5‐FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild‐type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). Journal of Clinical Oncology 2014;32(5s):Suppl; abstr LBA3.
Vincent 2011 {published data only}
-
- Vincent MD, Welch S, Soulieres D, Sanatani MS, Whiston F, Stitt L, et al. Randomized phase II trial of capecitabine (X) versus X plus erlotinib (E) in patients (pts) with metastatic colorectal cancer (mCRC): Differential impact of KRAS. Journal of Clinical Oncology 2011;29(15 Suppl):Abstr e14031.
Wasan COIN‐B 2014 {published data only}
-
- Wasan H, Meade AM, Adams R, Wilson R, Pugh C, Fisher D, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first‐line treatment of patients with KRAS wild‐type advanced colorectal cancer (COIN‐B): a randomised phase 2 trial. Lancet Oncology 2014;15(6):631‐9. - PMC - PubMed
Ye 2013 {published data only (unpublished sought but not used)}
-
- Xu J, Ye L, Ren L, Wei Y. A randomized, controlled trial of cetuximab plus chemotherapy for patients with KRAS wild‐type unresectable colorectal liver‐limited metastases. Annals of Oncology 2012;23 (Suppl 9):ix190; Abstract 557P. - PubMed
-
- Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. A randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild‐type unresectable colorectal liver‐limited metastases. Journal of Clinical Oncology 2013;31:1931‐8. - PubMed
-
- Ye LC, Zhong Y, Liu TS, Wei Y, Ren L, Zhu DX, et al. Impact of early tumor shrinkage on clinical outcome in KRAS wild‐type colorectal liver‐limited metastases treated with cetuximab plus chemotherapy: Lessons from a randomized controlled trial. Journal of Clinical Oncology 2013;31:Suppl; abstr 3610.
References to studies excluded from this review
Cunningham BOND 2004 {published data only}
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. New England Journal of Medicine 2004;351:337‐45. - PubMed
Liu 2015 {published data only}
-
- Liu Y, Luan L, Wang X. A randomized Phase II clinical study of combining panitumumab and bevacizumab, plus irinotecan, 5‐fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second‐line treatment for patients with metastatic colorectal cancer and KRAS mutation. Journal of OncoTargets and Therapy 2015;8:1061‐8. - PMC - PubMed
NCT00950820 {published data only}
-
- NCT00950820. Study to evaluate the effects of panitumumab if combined with chemotherapy for 2nd treatment of colorectal cancer (VOXEL). clinicaltrials.gov/ct2/show/NCT00950820 (first received 31 July 2009).
Personeni 2013 {published data only}
-
- Personeni N, Rimassa L, Verusio C, Barni S, Destro A, Raschioni C, et al. Prognostic factors in KRAS wild‐type (wt) metastatic colorectal cancer (mCRC) patients (pts) treated with biweekly cetuximab (C) plus irinotecan, fluorouracil, and leucovorin (FOLFIRI): A phase II study. Journal of Clinical Oncology 2013;31:Suppl; abstr e14611.
Primrose NEW EPOC 2014 {published data only}
-
- Primrose J, Falk S, Finch‐Jones M, Valle J, O'Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncology 2014;15(6):601‐11. - PubMed
Saltz BOND2 2007 {published data only}
-
- Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan‐refractory colorectal cancer: the BOND‐2 study. Journal of Clinical Oncology 2007;25(29):4557‐61. - PubMed
References to studies awaiting assessment
Hill 2015 {published data only}
-
- Hill A, Findlay M, Burge M, Jackson C, Alfonso PG, Samuel L, et al. Randomized phase II study of duligotuzumab + FOLFIRI versus cetuximab + FOLFIRI in 2nd‐line patients with KRAS wild‐type (wt) metastatic colorectal cancer (mCRC). Cancer Research 2015;75(15 Suppl):Abstr nr CT110. [DOI: 10.1158/1538-7445.AM2015-CT110] - DOI
Hiret 2016 {published data only}
-
- Hiret S, Borg C, Bertaut A, Bouche O, Adenis A, Deplanque G, et al. Bevacizumab or cetuximab plus chemotherapy after progression with bevacizumab plus chemotherapy in patients with wtKRAS metastatic colorectal cancer: A randomized phase II study (Prodige 18 –UNICANCER GI). Journal of Clinical Oncology 2016;34:Suppl; abstr 3514.
Kim 2016 {published data only}
-
- Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. An open label, randomized phase III trial evaluating the treatment (tx) effects of panitumumab (pmab) + best supportive care (BSC) versus BSC in chemorefractory wild‐type (WT) KRAS exon 2 metastatic colorectal cancer (mCRC) and in WT RAS mCRC. Journal of Clinical Oncology 2016;34:Suppl 4S; abstr 642.
-
- Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. Final results from a phase III trial evaluating panitumumab (pmab) + best supportive care (BSC) vs BSC in chemorefractory wild‐type (WT) KRAS exon 2 and WT RAS metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2016;34:Suppl; abstr 3536.
MACBETH 2016 {published data only}
-
- Antoniotti C, Cremolini C, Loupakis F, Bergamo F, Grande R, Tonini G, et al. Modified FOLFOXIRI (mFOLFOXIRI) plus cetuximab (cet), followed by cet or bevacizumab (bev) maintenance, in RAS/BRAF wild‐type (wt) metastatic colorectal cancer (mCRC): Results of the phase II randomized MACBETH trial by GONO. Journal of Clinical Oncology 2016;34:Suppl; abstr 3543.
-
- Cremolini C, Loupakis F, Salvatore L, Lonardi S, Battaglin F, Gamucci T, et al. Modified FOLFOXIRI plus cetuximab (cet) as induction treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): Preliminary results of the phase II randomized Macbeth trial by GONO group. Journal of Clinical Oncology 2014;32:5s:Abstr 3596.
References to ongoing studies
Ashwin 2014 {published data only}
ATOM {published data only}
-
- Emi Y, Muro K, Yamanaka T, Katayose Y, Uetake H, Sugihara K, et al. The ATOM trial: A multicenter, randomized phase II study of modified FOLFOX6 plus bevacizumab and modified FOLFOX6 plus cetuximab for colorectal cancer with liver‐limited metastases. Journal of Clinical Oncology 2016;34:Suppl 4S; abstr TPS777.
CAIRO5 {published data only}
-
- Huiskens J, Gulik TM, Lienden KP, Engelbrecht MRW, Meijer GA, Grieken NCT, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver‐only metastases: The randomized phase III CAIRO5 study of the Dutch Colorectal Cancer Group. Journal of Clinical Oncology 2015;33:Suppl; TPS3622. - PMC - PubMed
CREPAS {published data only}
-
- Study of medical treatment reactivity by the chemokine receptor (CXCR4) as 1st line treatment in patients with metastatic colorectal cancer (CREPAS). Ongoing study July 2012.
DEEPER {published data only}
-
- A Randomized Phase II Study to Investigate the Deepness of Response of FOLFOXIRI Plus Cetuximab (Erbitux) Versus FOLFOXIRI Plus Bevacizumab as the First‐line Therapy in Metastatic Colorectal Cancer Patients With RAS Wild‐type Tumors: DEEPER. Ongoing study August 2015.
FIRE‐4 {published data only}
-
- FIRE‐4: Randomised study of the efficacy of cetuximab rechallenge in patients with metastatic colorectal cancer (RAS wild‐type) responding to first‐line treatment with FOLFIRI plus cetuximab. Ongoing study March 2015.
FIRE‐4.5 {published data only}
-
- FIRE‐4.5. Ongoing study September 2016.
FOCETELD {published data only}
-
- FOCETELD. Ongoing study April 2012.
FOCULM {published data only}
-
- FOLFOXIRI With or Without Cetuximab as First‐line Treatment of Patients With Non‐resectable Liver‐Only Metastatic Colorectal Cancer (FOCULM). Ongoing study February 2014.
G13 study {published data only}
-
- Randomized Phase II Study of BSC vs Cetuximab vs Irinotecan and Cetuximab in Patients with KRAS codon G13D mutant Metastatic Colorectal Cancer (G13 study). Ongoing study September 2012. - PubMed
NCT00202787 {published data only}
-
- Open‐label, Phase II, Randomised, Pilot Study to Evaluate the Safety and Efficacy of Combination Therapy With Cetuximab and FOLFOX4 or FOLFOX4 Alone in Patients Colorectal Cancer and Initially Non‐resectable. Ongoing study February 2005.
NCT01442649 {published data only}
-
- Phase II, Multicentric Randomized Trial, Evaluating the Efficacy of Fluoropyrimidine‐based Standard Chemotherapy, Associated to Either Cetuximab or Bevacizumab, in KRAS Wild‐type Metastatic Colorectal Cancer Patients With Progressive Disease After Receiving First‐line Treatment With Bevacizumab. Ongoing study December 2010.
NCT01652482 {published data only}
-
- A Phase II, Multicenter, Open‐Label, Randomized Study Evaluating the Efficacy and Safety of MEHD7945A + FOLFIRI Versus Cetuximab + FOLFIRI in Second Line in Patients With KRAS Wildtype Metastatic Colorectal Cancer. Ongoing study October 2012.
NCT01991873 {published data only}
-
- NCT01991873. Maintenance therapy with 5‐FU/FA plus panitumumab vs. 5‐FU/FA alone after prior induction and re‐induction after progress for 1st‐line treatment of metastatic colorectal cancer (PanaMa). clinicaltrials.gov/ct2/show/NCT01991873 (first received 22 October 2013).
NCT02083653 {published data only}
-
- Open‐label, Randomized, Controlled, Multicenter Phase II Trial Investigating 2 Sym004 Doses Versus Investigator's Choice (Best Supportive Care, Capecitabine, 5‐FU) in Subjects With Metastatic Colorectal Cancer and Acquired Resistance to Anti‐EGFR Monoclonal Antibodies. Ongoing study March 2014.
NCT02394834 {published data only}
-
- An Exploratory Study of Treatment Sensitivity and Prognostic Factors in a Efficacy and Safety Study of mFOLFOX6 + Bevacizumab Versus mFOLFOX6 + Panitumumab Therapy in Patients With Chemotherapy‐naïve Unresectable Advanced or Recurrent Colorectal Cancer. Ongoing study May 2015.
PANIB {published data only}
-
- PANIB ‐ An open‐label, randomised, controlled, multi‐center, Phase II trial comparing Panitumumab versus Bevacizumab in combination with oxaliplatin ‐ 5 FU (FOLFOX) first‐line treatment according Ras Wild Type status for patients with metastatic unresectable colorectal cancer (mCRC). Ongoing study August 2014.
PARADIGM {published data only}
-
- Muro K, Uetake H, Tsuchihara K, Shitara K, Yamazaki K, Oki E, et al. PARADIGM study: A multicenter, randomized, phase III study of mFOLFOX6 plus panitumumab or bevacizumab as first‐line treatment in patients with RAS (KRAS/NRAS) wild‐type metastatic colorectal cancer. Journal of Clinical Oncology 2016;34:Suppl; abstr TPS3625.
-
- Yoshino T, Uetake H, Tsuchihara K, Shitara K, Yamazaki K, Oki E, et al. PARADIGM study: A multicenter, randomized, phase III study of 5‐fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus panitumumab or bevacizumab as first‐line treatment in patients with RAS (KRAS/NRAS) wild‐type metastatic colorectal cancer. Journal of Clinical Oncology 2016;34:Suppl 4S; abstr TPS776.
Peeters 2012 {published data only}
-
- Colorectal Cancer (CRC) Cetuximab Elderly Frail. Ongoing study April 2013.
TAILOR {published data only}
-
- An Open‐label, Randomized, Controlled, Multicenter Phase III Trial to Compare Cetuximab in Combination With FOLFOX‐4 Versus FOLFOX‐4 Alone in the First Line Treatment of Metastatic Colorectal Cancer in Chinese Subjects With RAS Wild‐type Status. Ongoing study August 2010.
TIME {published data only}
-
- Randomized Phase II Study of First‐line FOLFIRI Plus Cetuximab for 8 Cycles Followed by Either Single‐agent Cetuximab as Maintenance Therapy or Observation in Patients With Wild‐type KRAS and NRAS Metastatic Colorectal Cancer. Ongoing study February 2014.
UCGI 25 {published data only}
-
- Senellart H, Samalin E, Adenis A, Malka D, Francois E, Fouchardiere C, et al. UCGI 25: A multicentric randomized phase II trial evaluating dual targeting of the epidermal growth factor (EGFR) using the combination of cetuximab and afatinib versus cetuximab alone in patients (pts) wih chemotherapy refractory wtRAS metastatic colorectal cancer (mCRC). Journal of Clinical Oncology 2014;32;5s:Abstr TPS3666.
UMIN000005216 {published data only}
-
- Shitara K, Yonesaka K, Denda T, Moriwaki T, Tsuda M, Takano T, et al. A randomized multicenter phase II study of FOLFIRI plus either panitumumab (Pmab) or bevacizumab (Bmab) as second‐line treatment for wild‐type KRAS exon 2 metastatic colorectal cancer (mCRC) with exploratory biomarker analysis by liquid biopsy: WJOG6210G. Journal of Clinical Oncology 2016;34:Suppl; abstr 3567.
UMIN000006899 {published data only}
-
- Randomized phase II study of biweekly cetuximab versus panitumumab in patients not combination of irinotecan wild‐type KRAS metastatic colorectal cancer following treatment with fluoropyrimidine and oxaliplatin chemotherapy. Ongoing study December 2011.
Venook CALGB 80203 2006 {published data only}
-
- Venook A, Niedzwiecki D, Hollis D, Sutherland S, Goldberg R, Alberts S, et al. Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) ± cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. Journal of Clinical Oncology 2006;24(18 Suppl):3509.
VISNU‐2 {published data only}
-
- Influence of BRAF and PIK3K Status on the Efficacy of 5‐Fluorouracil/Leucovorin/Oxaliplatin (FOLFIRI) Plus Bevacizumab or Cetuximab in Patients With RAS Wild‐type Metastatic Colorectal Carcinoma and < 3 Circulating Tumor Cells (CTC) (VISNU‐2). Ongoing study July 2012.
VOLFI {published data only}
-
- Martens UM, Wessendorf S, Riera Knorrenschild J, Buechner‐Steudel P, Florschuetz A, Atzpodien J, et al. AIO‐KRK‐0109: A randomized phase II trial of panitumumab plus FOLFOXIRI or FOLFOXIRI alone as 1st‐line treatment in RAS‐wild‐type metastatic colorectal cancer (mCRC). European Journal of Cancer 2015;51:s344‐5.
WJOG6510G {published data only}
-
- Sakai D, Taniguchi H, Tamura T, Sugimoto N, Esaki T, Okuda H, et al. Randomized phase II study of panitumumab (Pmab) plus irinotecan (CPT‐11) versus cetuximab (Cmab) plus CPT‐11 in patients with KRAS wild‐type (WT) metastatic colorectal cancer (mCRC) following treatment with fluoropyrimidine, CPT‐11, and oxaliplatin (L‐OHP) chemotherapy: WJOG6510G. Journal of Clinical Oncology 2014;32 (5s):TPS3654.
Additional references
Andreyev 1998
-
- Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. Journal of the National Cancer Institute 1998;90(9):675‐84. - PubMed
Chan 2013 [pers comm]
-
- Chan DL. Request for EPIC KRAS substudy data [personal communication]. Email to: A Sobrero 23 September 2013.
Chan 2015
Chan 2015 [pers comm]
-
- Chan DL. Request for SAKK XELOX+C data ‐ extended RAS [personal communication]. Email to: M Borner 10 May 2015.
Chung 2005
-
- Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. Journal of Clinical Oncology 2005;23(9):1803‐10. - PubMed
Citri 2006
-
- Citri A, Yarden Y. EGF–ERBB signalling: towards the systems level. Nature Reviews Molecular Cell Biology 2006;7:505‐16. - PubMed
Cunningham 2004
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. New England Journal of Medicine 2004;351(4):337‐45. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Di Fiore 2007
-
- Fiore F, Pessot F, Lamy A, Charbonnier F, Sabourin J, Paillot B, et al. KRAS mutation is highly predictive of cetuximab resistance in metastatic colorectal cancer. Journal of Clinical Oncology (Meeting Abstracts) 2007;25(18 (Suppl)):10502.
Eisenhauer 2009
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228‐47. - PubMed
Favoni 2010
-
- Favoni RE, Pattarozzi A, Lo Casto M, Barbieri F, Gatti M, Paleari L, et al. Gefitinib targets EGFR dimerization and ERK1/2 phosphorylation to inhibit pleural mesothelioma cell proliferation. Current Cancer Drug Targets 2010;10(2):176‐91. - PubMed
Feng 2001
-
- Feng J, Hua F, Shuo R, Chongfeng G, Huimian X, Nakajima T, et al. Upregulation of non‐mutated H‐ras and its upstream and downstream signaling proteins in colorectal cancer. Oncology Reports 2001;8(6):1409‐13. - PubMed
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [PUBMED: 25220842] - PubMed
Giantonio 2007
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 2007;25(12):1539‐44. - PubMed
Graham 2014
-
- Graham CN, Hechmati G, Hjelmgren J, Liege F, Lanier J, Knox H, et al. Cost‐effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first‐line treatment of patients with wild‐type RAS metastatic colorectal cancer. European Journal of Cancer 2014;50(16):2791‐801. - PubMed
Grothey 2004
-
- Grothey A, Sargent D, Goldberg RM, Schmoll H. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil‐leucovorin, irinotecan, and oxaliplatin in the course of treatment. Journal of Clinical Oncology 2014;22(7):1209‐14. - PubMed
Herbertson 2009
Herbst 2004
-
- Herbst RS. Review of epidermal growth factor receptor biology.. International Journal of Radiation Oncology • Biology • Physics 2004;59(Suppl 2):21‐6. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hurwitz 2005
-
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first line metastatic colorectal cancer. Journal of Clinical Oncology 2005;23(15):3502‐8. - PubMed
Jonker 2007
-
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. New England Journal of Medicine 2007;357(20):2040‐8. - PubMed
Khattak 2015
-
- Khattak MA, Martin H, Davidson A, Phillips M. Role of first‐line anti‐epidermal growth factor receptor therapy compared with anti‐vascular endothelial growth factor therapy in advanced colorectal cancer: a meta‐analysis of randomized clinical trials. Clinical Colorectal Cancer 2015;14(2):81‐90. - PubMed
Kumachev 2014
-
- Kumachev A, Yan M, Berry SR, Ko YJ, Martinez MCR, Shah K. A comparison of biologics in first‐line advanced colorectal cancer: A Bayesian network meta‐analysis of EGFR inhibitors and bevacizumab. Journal of Clinical Oncology 2014;32:Suppl 3; abstr 543.
Lee 2009
-
- Lee SC, Xu X, Lim YW, Iau P, Sukri N, Lim SE, et al. Chemotherapy‐induced tumor gene expression changes in human breast cancers. Pharmacogenetics and Genomics 2009;19(3):181‐92. - PubMed
Lievre 2006
-
- Lievre A, Bachet JB, Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Research 2006;66(8):3992‐5. - PubMed
Mayer 1993
-
- Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer 1993;71(8):2454‐60. - PubMed
Mendelsohn 2000
-
- Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene 2000;19(56):6550‐65. - PubMed
Messa 1998
-
- Messa C, Russo F, Caruso MG, Leo A. EGF, TGF‐alpha, and EGF‐R in human colorectal adenocarcinoma. Acta Oncologica 1998;37(3):285‐9. - PubMed
Mohan 2014
Mok 2009
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. New England Journal of Medicine 2009;361:947‐57. [PUBMED: 19692680] - PubMed
NIH 2010
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf (accessed prior to 30 May 2017).
Normanno 2006
-
- Normanno N, Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signalling in cancer. Gene 2006;366(1):2‐16. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] - PubMed
Rajagopalan 2002
-
- Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch‐repair status. Nature 2002;418(6901):934. - PubMed
Rauw 2012
-
- Rauw J, Ennis M, Krzyzanowska MK, Sridhar SS. Does the addition of molecular targeted therapy to standard treatments lead to better or worse outcomes overall ‐ a systematic review of EGFR‐targeted therapies used in combination with standard treatments. Journal of Clinical Oncology 2012;30:Suppl; abstr 2572.
Salomon 1995
-
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor‐related peptides and their receptors in human malignancies. Critical Reviews in Oncology/Hematology 1995;19(3):183‐232. - PubMed
Scartozzi 2004
-
- Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: Implications for treatment with EGFR‐targeted monoclonal antibodies. Journal of Clinical Oncology 2004;22(23):4772‐8. - PubMed
Schrag 2015
-
- Schrag D, Dueck AC, Naughton MJ, Niedzwiceki D, Earle C, Shaw JE, et al. Cost of chemotherapy for metastatic colorectal cancer with either bevacizumab or cetuximab: Economic analysis of CALGB/SWOG 80405. Journal of Clinical Oncology 2015;33(Suppl):Abstr 6504.
Schünemann 2009
-
- Schünemann H, Brozek J, Oxman A, editors of The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations Version 3.2 [updated March 2009]. Available from guidelinedevelopment.org/handbook.
Sorich 2015
-
- Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti‐EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta‐analysis of randomized, controlled trials.. Annals of Oncology 2015;26(1):13‐21. - PubMed
Vale 2012
-
- Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, et al. Does anti‐EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta‐analysis. Cancer Treatment Reviews 2012;38(6):618‐25. - PubMed
van Cruijsen 2005
-
- Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptors and angiogenesis: Opportunities for combined anticancer strategies. International Journal of Cancer 2005;117(6):883‐8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

