Strongly Reducing, Visible-Light Organic Photoredox Catalysts as Sustainable Alternatives to Precious Metals
- PMID: 28654171
- PMCID: PMC5941304
- DOI: 10.1002/chem.201702926
Strongly Reducing, Visible-Light Organic Photoredox Catalysts as Sustainable Alternatives to Precious Metals
Abstract
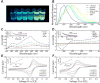
Photoredox catalysis is a versatile approach for the construction of challenging covalent bonds under mild reaction conditions, commonly using photoredox catalysts (PCs) derived from precious metals. As such, there is need to develop organic analogues as sustainable replacements. Although several organic PCs have been introduced, there remains a lack of strongly reducing, visible-light organic PCs. Herein, we establish the critical photophysical and electrochemical characteristics of both a dihydrophenazine and a phenoxazine system that enables their success as strongly reducing, visible-light PCs for trifluoromethylation reactions and dual photoredox/nickel-catalyzed C-N and C-S cross-coupling reactions, both of which have been historically exclusive to precious metal PCs.
Keywords: organocatalysis; photocatalysis; photochemistry; radicals; sustainable chemistry.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Adachi C, Baldo MA, Thompson ME, Forrest SR. J Appl. Phys. 2001;90:5048–5051.
-
- Allardyce CS, Dyson PJ. Platin. Met. Rev. 2001;45:62–69.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

