Preparation and Evaluation of Potent Pentafluorosulfanyl-Substituted Anti-Tuberculosis Compounds
- PMID: 28654200
- PMCID: PMC5603227
- DOI: 10.1002/cmdc.201700170
Preparation and Evaluation of Potent Pentafluorosulfanyl-Substituted Anti-Tuberculosis Compounds
Abstract
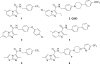
The global fight to stop tuberculosis (TB) remains a great challenge, particularly with the increase in drug-resistant strains and a lack of funding to support the development of new treatments. To bolster a precarious drug pipeline, we prepared a focused panel of eight pentafluorosulfanyl (SF5 ) compounds which were screened for their activity against Mycobacterium tuberculosis (Mtb) H37Rv in three different assay conditions and media. All eight compounds had sub-micromolar potency, and four displayed MICs <100 nm. Seven compounds were evaluated against non-replicating and mono-drug-resistant Mtb, and for their ability to inhibit Mtb within the macrophage. The greatest potency was observed against intracellular Mtb (MIC <10 nm for three compounds), which is often the most challenging to target. In general, the SF5 -bearing compounds were very similar to their CF3 counterparts, with the major differences observed being their in vitro ADME properties. Two SF5 -bearing compounds were found to have greater protein binding than their corresponding CF3 counterparts, but were also less metabolized in human microsomes, resulting in longer half-lives.
Keywords: Mycobacterium tuberculosis; drug resistance; imidazo[1,2-a]pyridines; imidazo[2,1-b]thiazoles; pentafluorosulfanyl.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- World Health Organization (WHO) Global Tuberculosis control WHO report 2016. 2016 http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf (Last accessed: December 1, 2016)
-
- Stucki D, Brites D, Jeljeli L, Coscolla M, Liu Q, Trauner A, Fenner L, Rutaihwa L, Borrell S, Luo T, Gao Q, Kato-Maeda M, Ballif M, Egger M, Macedo R, Mardassi H, Moreno M, Vilanova GT, Fyfe J, Globan M, Thomas J, Jamieson F, Guthrie JL, Asante-Poku A, Yeboah-Manu D, Wampande E, Ssengooba W, Joloba M, Boom WH, Basu I, Bower J, Saraiva M, Vasconcellos SEG, Suffys P, Koch A, Wilkinson R, Gail-Bekker L, Malla B, Ley SD, Beck HP, de Jong BC, Toit K, Sanchez-Padilla E, Bonnet M, Gil-Brusola A, Frank M, Beng VNP, Eisenach K, Alani I, Ndung’u PW, Revathi G, Gehre F, Akter S, Ntoumi F, Stewart-Isherwood L, Ntinginya NE, Rachow A, Hoelscher M, Cirillo DM, Skenders G, Hoffner S, Bakonyte D, Stakenas P, Diel R, Crudu V, Moldovan O, Al-Hajoj S, Otero L, Barletta F, Carter EJ, Diero L, Supply P, Comas I, Niemann S, Gagneux S. Nat Genet. 2016;48:1535–1543. - PMC - PubMed
-
- Li H, Gu Y, Wang X, Zhu T, Duan H, Ma Y, Huang H, Javid B. J Clin Mcrobiol. 2015;53:2781–2784. - PMC - PubMed
- Parida SK, Axelsson-Robertson R, Rao MV, Singh N, Master I, Lutckii A, Keshavjee S, Andersson J, Zumla A, Maeurer M. J Intern Med. 2015;277:388–405. - PubMed
- Palomino JC, Martin A. Future Microbiol. 2016;11:539–47. - PubMed
-
- Moraski GC, Markley LD, Hipskind PA, Boshoff H, Cho S, Franzblau SG, Miller MJ. ACS Med Chem Lett. 2011;2:466–470. - PMC - PubMed
- Ollinger J, Bailey MA, Moraski GC, Casey A, Florio S, Alling T, Miller MJ, Parish T. PloS One. 2013;8:e60531. - PMC - PubMed
- Moraski GC, Miller PA, Bailey MA, Ollinger J, Parish T, Boshoff HI, Cho S, Anderson JR, Mulugeta S, Franzblau SG, Miller MJ. ACS Infect Dis. 2015;1:85–90. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

