Brain and Brown Adipose Tissue Metabolism in Transgenic Tg2576 Mice Models of Alzheimer Disease Assessed Using 18F-FDG PET Imaging
- PMID: 28654383
- PMCID: PMC5470140
- DOI: 10.1177/1536012117704557
Brain and Brown Adipose Tissue Metabolism in Transgenic Tg2576 Mice Models of Alzheimer Disease Assessed Using 18F-FDG PET Imaging
Abstract
Objective: Imaging animal models of Alzheimer disease (AD) is useful for the development of therapeutic drugs and understanding AD. Transgenic Swedish hAPPswe Tg2576 mice are a good model of β-amyloid plaques. We report 18F-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) imaging of brain and intrascapular brown adipose tissue (IBAT) in transgenic mice 2576 (Tg2576) and wild-type (WT) mice.
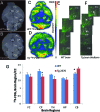
Methods: Transgenic Tg2576 mice and WT mice, >18 months were injected intraperitonally with ≈ 25 to 30 MBq 18F-FDG while awake. After 60 minutes, they were anesthetized with isoflurane (2.5%) and imaged with Inveon MicroPET. Select mice were killed, imaged ex vivo, and 20 µm sections cut for autoradiography. 18F-FDG uptake in brain and IBAT PET and brain autoradiographs were analyzed.
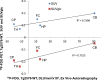
Results: Fasting blood glucose levels averaged 120 mg/dL for WT and 100 mg/dL for Tg2576. Compared to WT, Tg2576 mice exhibited a decrease in SUVglc in the various brain regions. Average reductions in the cerebrum regions were as high as -20%, while changes in cerebellum were -3%. Uptake of 18F-FDG in IBAT decreased by -60% in Tg2576 mice and was found to be significant. Intrascapular brown adipose tissue findings in Tg2576 mice are new and not previously reported. Use of blood glucose for PET data analysis and corpus callosum as reference region for autoradiographic analysis were important to detect change in Tg2576 mice.
Conclusion: Our results suggest that 18F-FDG uptake in the Tg2576 mice brain show 18F-FDG deficits only when blood glucose is taken into consideration.
Keywords: Alzheimer disease; PET imaging; brain; brown adipose tissue; glucose metabolism; norepinephrine; transgenic mice 2576.
Conflict of interest statement
Figures



References
-
- Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. - PubMed
-
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56(9):965–973. - PubMed
-
- Chapman PF, White GL, Jones MW, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2(3):271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

