Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer
- PMID: 28654894
- PMCID: PMC5564823
- DOI: 10.18632/oncotarget.18442
Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer
Abstract
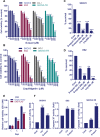
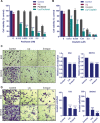
Ovarian cancer is the deadliest of all gynecologic cancers. Despite success with initial chemotherapy, the majority of patients relapse with an incurable disease. Development of chemotherapy resistance is a major factor for poor long-term survival in ovarian cancer. The biological effects of estrogens are mediated by estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). Emerging evidence suggests that ovarian cancer cells express ERβ that functions as a tumor suppressor; however, the clinical utility of ERβ agonists in ovarian cancer remains elusive. We tested the utility of two natural ERβ agonists liquiritigenin (Liq), which is isolated from Glycyrrhiza uralensis and S-equol, which is isolated from soy isoflavone daidzein, for treating ovarian cancer. Both natural ERβ ligands had significant growth inhibition in cell viability and survival assays, reduced migration and invasion, and promoted apoptosis. Further, ERβ agonists showed tumor suppressive functions in therapy-resistant ovarian cancer model cells and sensitized ovarian cancer cells to cisplatin and paclitaxel treatment. Global RNA-Seq analysis revealed that ERβ agonists modulate several tumor suppressive pathways, including downregulation of the NF-κB pathway. Immunoprecipitation assays revealed that ERβ interacts with p65 subunit of NF-κB and ERβ overexpression reduced the expression of NF-κB target genes. In xenograft assays, ERβ agonists reduced tumor growth and promoted apoptosis. Collectively, our findings demonstrated that natural ERβ agonists have the potential to significantly inhibit ovarian cancer cell growth by anti-inflammatory and pro-apoptotic actions, and natural ERβ agonists represent novel therapeutic agents for the management of ovarian cancer.
Keywords: NF-κB; natural ERβ agonists; ovarian cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Effect of estrogen receptor β agonists on proliferation and gene expression of ovarian cancer cells.BMC Cancer. 2017 May 8;17(1):319. doi: 10.1186/s12885-017-3246-0. BMC Cancer. 2017. PMID: 28482871 Free PMC article.
-
Differential activation of NF-κB signaling is associated with platinum and taxane resistance in MyD88 deficient epithelial ovarian cancer cells.Int J Biochem Cell Biol. 2015 Apr;61:90-102. doi: 10.1016/j.biocel.2015.02.001. Epub 2015 Feb 11. Int J Biochem Cell Biol. 2015. PMID: 25681684
-
Activation of estrogen receptor beta signaling reduces stemness of glioma stem cells.Stem Cells. 2021 May;39(5):536-550. doi: 10.1002/stem.3337. Epub 2021 Feb 1. Stem Cells. 2021. PMID: 33470499
-
Ovarian actions of estrogen receptor-β: an update.Semin Reprod Med. 2012 Jan;30(1):32-8. doi: 10.1055/s-0031-1299595. Epub 2012 Jan 23. Semin Reprod Med. 2012. PMID: 22271292 Review.
-
Cancer therapy using natural ligands that target estrogen receptor beta.Chin J Nat Med. 2015 Nov;13(11):801-807. doi: 10.1016/S1875-5364(15)30083-2. Chin J Nat Med. 2015. PMID: 26614454 Free PMC article. Review.
Cited by
-
Estrogen receptor β exerts tumor suppressive effects in prostate cancer through repression of androgen receptor activity.PLoS One. 2020 May 15;15(5):e0226057. doi: 10.1371/journal.pone.0226057. eCollection 2020. PLoS One. 2020. PMID: 32413024 Free PMC article.
-
Estrogen-Receptor Expression and Function in Female Reproductive Disease.Cells. 2019 Sep 21;8(10):1123. doi: 10.3390/cells8101123. Cells. 2019. PMID: 31546660 Free PMC article. Review.
-
Soy-Induced Fecal Metabolome Changes in Ovariectomized and Intact Female Rats: Relationship with Cardiometabolic Health.Sci Rep. 2018 Nov 15;8(1):16896. doi: 10.1038/s41598-018-35171-3. Sci Rep. 2018. PMID: 30442926 Free PMC article.
-
Estrogen Receptors Alpha and Beta in Acute Myeloid Leukemia.Cancers (Basel). 2020 Apr 8;12(4):907. doi: 10.3390/cancers12040907. Cancers (Basel). 2020. PMID: 32276421 Free PMC article. Review.
-
Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality.Oncogenesis. 2018 Feb 9;7(2):15. doi: 10.1038/s41389-018-0027-9. Oncogenesis. 2018. PMID: 29422491 Free PMC article.
References
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. - PubMed
-
- Petrillo M, Nero C, Amadio G, Gallo D, Fagotti A, Scambia G. Targeting the hallmarks of ovarian cancer: the big picture. Gynecol Oncol. 2016;142:176–183. - PubMed
-
- Dutta S, Wang FQ, Phalen A, Fishman DA. Biomarkers for ovarian cancer detection and therapy. Cancer Biol Ther. 2010;9:668–677. - PubMed
-
- Grisham RN, Hyman DM, Iyer G. Targeted therapies for treatment of recurrent ovarian cancer. Clin Adv Hematol Oncol. 2014;12:158–162. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

