TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis
- PMID: 28655204
- PMCID: PMC6251650
- DOI: 10.1093/eurheartj/ehx315
TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis
Abstract
Aims: The recent failures of HDL-raising therapies have underscored our incomplete understanding of HDL biology. Therefore there is an urgent need to comprehensively investigate HDL metabolism to enable the development of effective HDL-centric therapies. To identify novel regulators of HDL metabolism, we performed a joint analysis of human genetic, transcriptomic, and plasma HDL-cholesterol (HDL-C) concentration data and identified a novel association between trafficking protein, kinesin binding 2 (TRAK2) and HDL-C concentration. Here we characterize the molecular basis of the novel association between TRAK2 and HDL-cholesterol concentration.
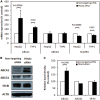
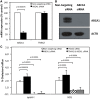
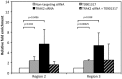
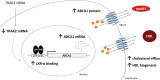
Methods and results: Analysis of lymphocyte transcriptomic data together with plasma HDL from the San Antonio Family Heart Study (n = 1240) revealed a significant negative correlation between TRAK2 mRNA levels and HDL-C concentration, HDL particle diameter and HDL subspecies heterogeneity. TRAK2 siRNA-mediated knockdown significantly increased cholesterol efflux to apolipoprotein A-I and isolated HDL from human macrophage (THP-1) and liver (HepG2) cells by increasing the mRNA and protein expression of the cholesterol transporter ATP-binding cassette, sub-family A member 1 (ABCA1). The effect of TRAK2 knockdown on cholesterol efflux was abolished in the absence of ABCA1, indicating that TRAK2 functions in an ABCA1-dependent efflux pathway. TRAK2 knockdown significantly increased liver X receptor (LXR) binding at the ABCA1 promoter, establishing TRAK2 as a regulator of LXR-mediated transcription of ABCA1.
Conclusion: We show, for the first time, that TRAK2 is a novel regulator of LXR-mediated ABCA1 expression, cholesterol efflux, and HDL biogenesis. TRAK2 may therefore be an important target in the development of anti-atherosclerotic therapies.
Keywords: ABCA1; Atherosclerosis; Cholesterol; Genetics; HDL; TRAK2.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2017. For permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Making sense of a seemingly odd connection.Eur Heart J. 2017 Dec 21;38(48):3588-3589. doi: 10.1093/eurheartj/ehx506. Eur Heart J. 2017. PMID: 29020393 Free PMC article. No abstract available.
References
-
- Kingwell BA, Chapman MJ, Kontush A, Miller NE.. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov 2014;13:445–464. - PubMed
-
- Cuchel M, Rader DJ.. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 2006;113:2548–2555. - PubMed
-
- Zhao C, Dahlman-Wright K.. Liver X receptor in cholesterol metabolism. J Endocrinol 2010;204:233–240. - PubMed
-
- Sabol SL, Brewer HB Jr, Santamarina-Fojo S.. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. J Lipid Res 2005;46:2151–2167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

