Protease-activated receptor-1 activation by granzyme B causes neurotoxicity that is augmented by interleukin-1β
- PMID: 28655310
- PMCID: PMC5488439
- DOI: 10.1186/s12974-017-0901-y
Protease-activated receptor-1 activation by granzyme B causes neurotoxicity that is augmented by interleukin-1β
Abstract
Background: The cause of neurodegeneration in progressive forms of multiple sclerosis is unknown. We investigated the impact of specific neuroinflammatory markers on human neurons to identify potential therapeutic targets for neuroprotection against chronic inflammation.
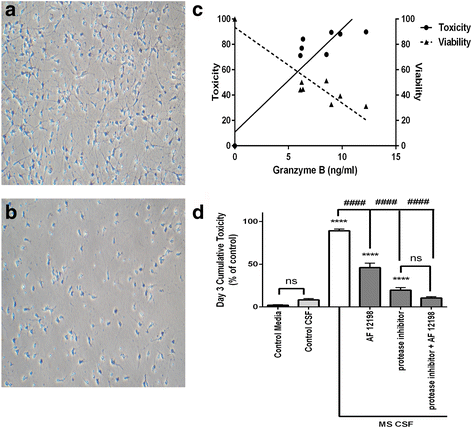
Methods: Surface immunocytochemistry directly visualized protease-activated receptor-1 (PAR1) and interleukin-1 (IL-1) receptors on neurons in human postmortem cortex in patients with and without neuroinflammatory lesions. Viability of cultured neurons was determined after exposure to cerebrospinal fluid from patients with progressive multiple sclerosis or purified granzyme B and IL-1β. Inhibitors of PAR1 activation and of PAR1-associated second messenger signaling were used to elucidate a mechanism of neurotoxicity.
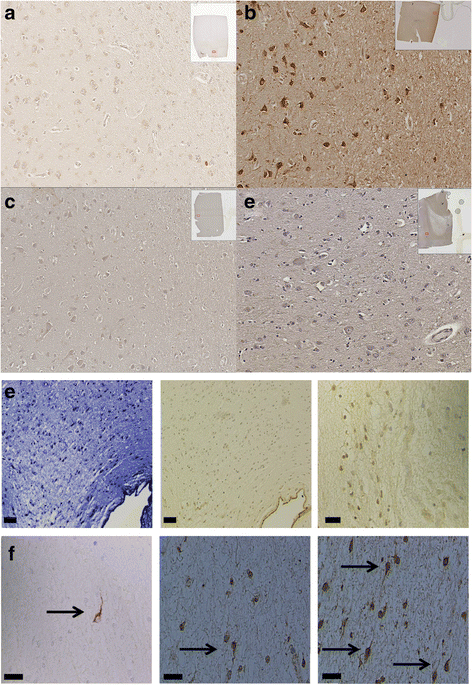
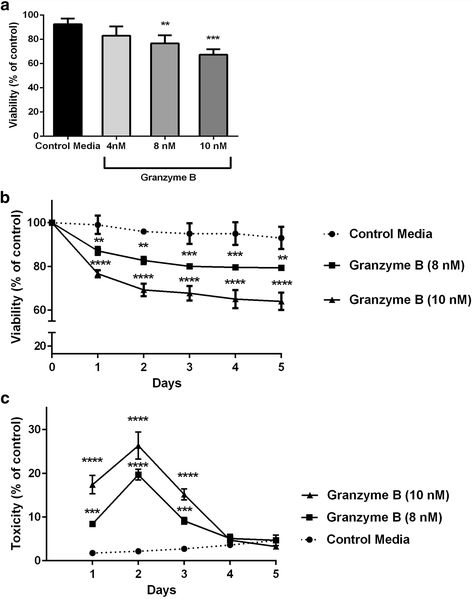
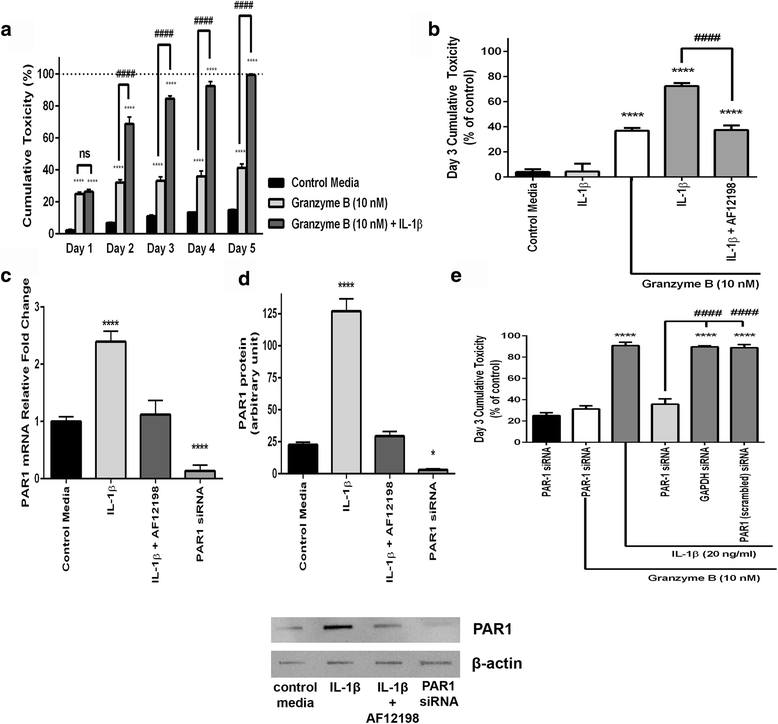
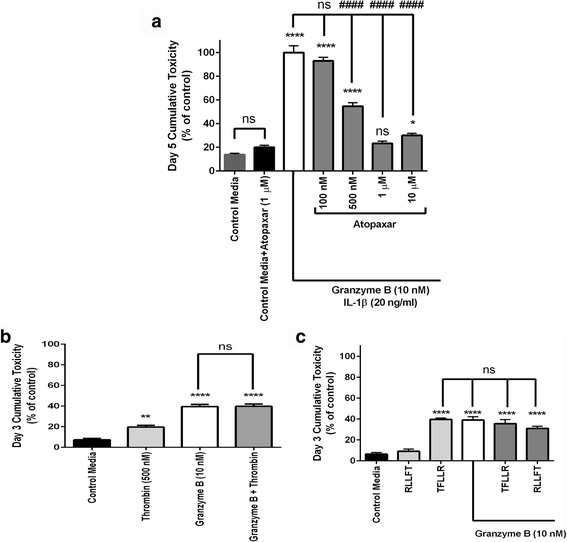
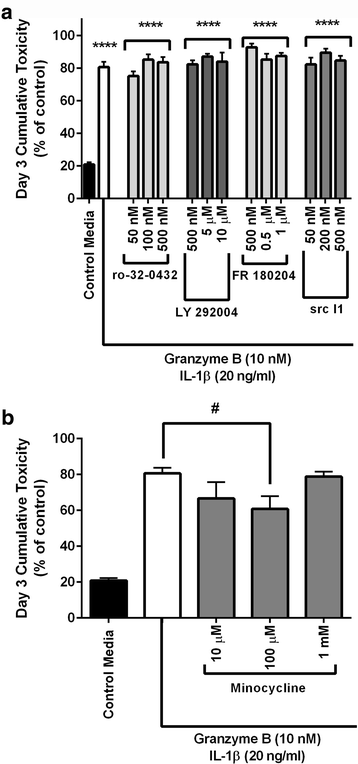
Results: Immunohistochemistry of human post-mortem brain tissue demonstrated cells expressing higher amounts of PAR1 near and within subcortical lesions in patients with multiple sclerosis compared to control tissue. Human cerebrospinal fluid samples containing granzyme B and IL-1β were toxic to human neuronal cultures. Granzyme B was neurotoxic through activation of PAR1 and subsequently the phospholipase Cβ-IP3 second messenger system. Inhibition of PAR1 or IP3 prevented granzyme B toxicity. IL-1β enhanced granzyme B-mediated neurotoxicity by increasing PAR1 expression.
Conclusions: Neurons within the inflamed central nervous system are imperiled because they express more PAR1 and are exposed to a neurotoxic combination of both granzyme B and IL-1β. The effects of these inflammatory mediators may be a contributing factor in the progressive brain atrophy associated with neuroinflammatory diseases. Knowledge of how exposure to IL-1β and granzyme B act synergistically to cause neuronal death yields potential novel neuroprotective treatments for neuroinflammatory diseases.
Keywords: Granzyme B; Interleukin-1; Multiple sclerosis; Neuroinflammation; Neurotoxicity; Protease-activated receptor.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

