Treatment of severe hospital-acquired and ventilator-associated pneumonia: a systematic review of inclusion and judgment criteria used in randomized controlled trials
- PMID: 28655326
- PMCID: PMC5488424
- DOI: 10.1186/s13054-017-1755-5
Treatment of severe hospital-acquired and ventilator-associated pneumonia: a systematic review of inclusion and judgment criteria used in randomized controlled trials
Abstract
Background: Hospital-acquired and ventilator-associated pneumonia (HAP/VAP) are often selected for randomized clinical trials (RCTs) aiming at new drug approval. Guidelines for the design of such RCTs have been repeatedly updated by regulatory agencies. We hypothesized that large variability in the enrolled populations, the endpoints assessed and the HAP/VAP definition criteria may impact the results of these studies, and addressed this through a systematic review of HAP/VAP RCTs.
Methods: A search (Pubmed-Embase-ICAAC-ECCMID) of all RCTs published between 1994 and 2016 comparing antimicrobial treatment for HAP/VAP in the intensive care unit was conducted. The populations enrolled, inclusion/exclusion criteria, statistical design and endpoints assessed were recorded. All unpublished RCTs recorded on the ClinicalTrials.gov registry were also screened.
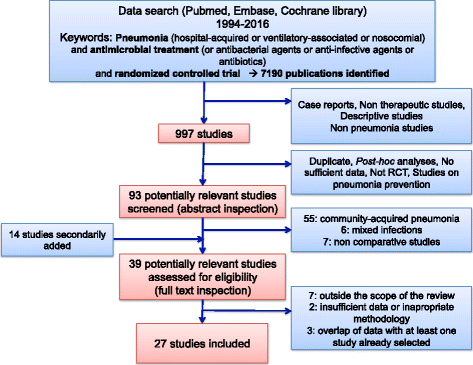
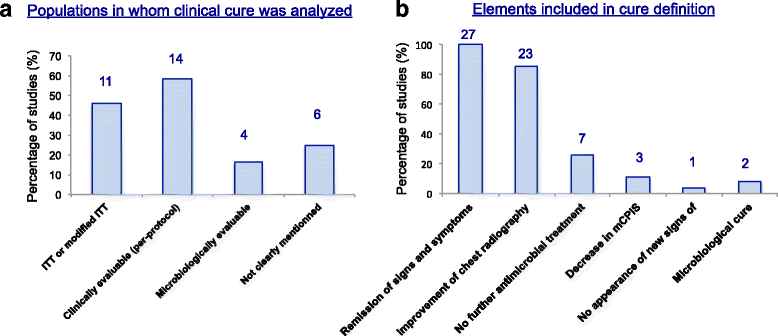
Results: From the 93 abstracts reviewed, 39 potentially relevant studies were inspected, leading to 27 studies being included. As expected, illness severity or the proportion with VAP (27-100%) differed greatly among the enrolled populations. The HAP/VAP definition used various clinical and biological criteria, and only 55% of studies required a microbiological sample. The mandatory duration of prior hospital stay was variable; the mechanical ventilation duration was an inclusion criterion in only 41% of VAP studies. Nine studies had non-inferiority design, but nine studies (33%) did not have a pre-specified statistical hypothesis. Clinical cure was the primary endpoint in 24 studies, but was recorded in several populations or as the co-primary endpoint in 13 studies. The definition of clinical cure and the timing of its assessment greatly differed. This variability slightly improved over time but remained significant in the 13 registered but currently unpublished RCTs that we screened.
Conclusion: Our study provides a description of populations and endpoints of RCTs evaluating antimicrobials for treatment of HAP/VAP in the ICU. There was significant heterogeneity in enrollment criteria, endpoints and statistical design, which may influence the ability of studies to demonstrate differences between studied drugs.
Keywords: Endpoints; Hospital-acquired pneumonia treatment; Inclusion criteria; Randomized controlled trials; Systematic review; Ventilator-acquired pneumonia treatment.
Figures

Comment in
-
Adopting a smart toothbrush with artificial intelligence may improve oral care in patients admitted to the intensive care unit.Crit Care. 2019 Jun 18;23(1):223. doi: 10.1186/s13054-019-2512-8. Crit Care. 2019. PMID: 31215441 Free PMC article. No abstract available.
References
-
- Guidance for Industry Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia: Developing Drugs for Treatment. In: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2014.
-
- Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. In: European Medical Agency; 2013.
-
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

