RBM25 is a global splicing factor promoting inclusion of alternatively spliced exons and is itself regulated by lysine mono-methylation
- PMID: 28655759
- PMCID: PMC5555197
- DOI: 10.1074/jbc.M117.784371
RBM25 is a global splicing factor promoting inclusion of alternatively spliced exons and is itself regulated by lysine mono-methylation
Abstract
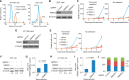
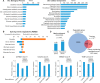
In eukaryotes, precursor mRNA (pre-mRNA) splicing removes non-coding intron sequences to produce mature mRNA. This removal is controlled in part by RNA-binding proteins that regulate alternative splicing decisions through interactions with the splicing machinery. RNA binding motif protein 25 (RBM25) is a putative splicing factor strongly conserved across eukaryotic lineages. However, the role of RBM25 in global splicing regulation and its cellular functions are unknown. Here we show that RBM25 is required for the viability of multiple human cell lines, suggesting that it could play a key role in pre-mRNA splicing. Indeed, transcriptome-wide analysis of splicing events demonstrated that RBM25 regulates a large fraction of alternatively spliced exons throughout the human genome. Moreover, proteomic analysis indicated that RBM25 interacts with components of the early spliceosome and regulators of alternative splicing. Previously, we identified an RBM25 species that is mono-methylated at lysine 77 (RBM25K77me1), and here we used quantitative mass spectrometry to show that RBM25K77me1 is abundant in multiple human cell lines. We also identified a region of RBM25 spanning Lys-77 that binds with high affinity to serine- and arginine-rich splicing factor 2 (SRSF2), a crucial protein in exon definition, but only when Lys-77 is unmethylated. Together, our findings uncover a pivotal role for RBM25 as an essential regulator of alternative splicing and reveal a new potential mechanism for regulation of pre-mRNA splicing by lysine methylation of a splicing factor.
Keywords: RNA splicing; alternative splicing; protein methylation; protein-protein interaction; proteomics.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures


References
-
- Green M. R. (1986) Pre-mRNA splicing. Annu. Rev. Genet. 20, 671–708 - PubMed
-
- McKeown M. (1992) Alternative mRNA splicing. Annu. Rev. Cell Biol. 8, 133–155 - PubMed
-
- Black D. L. (2000) Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103, 367–370 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

