Isolation and structure-function characterization of a signaling-active rhodopsin-G protein complex
- PMID: 28655769
- PMCID: PMC5572916
- DOI: 10.1074/jbc.M117.797100
Isolation and structure-function characterization of a signaling-active rhodopsin-G protein complex
Abstract
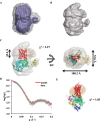
The visual photo-transduction cascade is a prototypical G protein-coupled receptor (GPCR) signaling system, in which light-activated rhodopsin (Rho*) is the GPCR catalyzing the exchange of GDP for GTP on the heterotrimeric G protein transducin (GT). This results in the dissociation of GT into its component αT-GTP and β1γ1 subunit complex. Structural information for the Rho*-GT complex will be essential for understanding the molecular mechanism of visual photo-transduction. Moreover, it will shed light on how GPCRs selectively couple to and activate their G protein signaling partners. Here, we report on the preparation of a stable detergent-solubilized complex between Rho* and a heterotrimer (GT*) comprising a GαT/Gαi1 chimera (αT*) and β1γ1 The complex was formed on native rod outer segment membranes upon light activation, solubilized in lauryl maltose neopentyl glycol, and purified with a combination of affinity and size-exclusion chromatography. We found that the complex is fully functional and that the stoichiometry of Rho* to GαT* is 1:1. The molecular weight of the complex was calculated from small-angle X-ray scattering data and was in good agreement with a model consisting of one Rho* and one GT*. The complex was visualized by negative-stain electron microscopy, which revealed an architecture similar to that of the β2-adrenergic receptor-GS complex, including a flexible αT* helical domain. The stability and high yield of the purified complex should allow for further efforts toward obtaining a high-resolution structure of this important signaling complex.
Keywords: 7-helix receptor; EM; G protein-coupled receptor (GPCR); G-protein; SAXS; photo-transduction; rhodopsin; signal transduction; structural biology; transducin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Ma P., and Zemmel R. (2002) Value of novelty? Nat. Rev. Drug Discov. 1, 571–572 - PubMed
-
- De Lean A., Stadel J. M., and Lefkowitz R. J. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 255, 7108–7117 - PubMed
-
- Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., and Kobilka B. K. (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 - PubMed
-
- Xu S., and Fischetti R. F. (2007) Design and performance of a compact collimator on GM/CA-CAT at the advanced photon source. Proc. SPIE. 6665, 1–8
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

