Peptides derived from MARCKS block coagulation complex assembly on phosphatidylserine
- PMID: 28655899
- PMCID: PMC5487340
- DOI: 10.1038/s41598-017-04494-y
Peptides derived from MARCKS block coagulation complex assembly on phosphatidylserine
Abstract
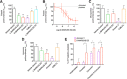
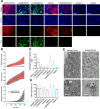
Blood coagulation involves activation of platelets and coagulation factors. At the interface of these two processes resides the lipid phosphatidylserine. Activated platelets expose phosphatidylserine on their outer membrane leaflet and activated clotting factors assemble into enzymatically active complexes on the exposed lipid, ultimately leading to the formation of fibrin. Here, we describe how small peptide and peptidomimetic probes derived from the lipid binding domain of the protein myristoylated alanine-rich C-kinase substrate (MARCKS) bind to phosphatidylserine exposed on activated platelets and thereby inhibit fibrin formation. The MARCKS peptides antagonize the binding of factor Xa to phosphatidylserine and inhibit the enzymatic activity of prothrombinase. In whole blood under flow, the MARCKS peptides colocalize with, and inhibit fibrin cross-linking, of adherent platelets. In vivo, we find that the MARCKS peptides circulate to remote injuries and bind to activated platelets in the inner core of developing thrombi.
Conflict of interest statement
H.Y., R.T., and N.K. have filed patent applications related to this work.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

