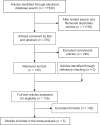
Efficacy of Human Papillomavirus L1 Protein Vaccines (Cervarix and Gardasil) in Reducing the Risk of Cervical Intraepithelial Neoplasia: A Meta-analysis
- PMID: 28656100
- PMCID: PMC5474905
- DOI: 10.4103/ijpvm.IJPVM_413_16
Efficacy of Human Papillomavirus L1 Protein Vaccines (Cervarix and Gardasil) in Reducing the Risk of Cervical Intraepithelial Neoplasia: A Meta-analysis
Abstract
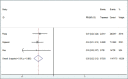
Human papillomavirus (HPV) can induce cervical intraepithelial neoplasia (CIN). Vaccination against HPV can play an important role in CIN prevention. This study aims to estimate the efficacy of L1 protein vaccines (Cervarix and Gardasil) in CIN 1, 2, 3 risk reduction using meta-analysis. Relevant articles were identified by two independent researchers searching international databanks. After application of inclusion/exclusion criteria and quality assessment, eligible articles were entered into the final meta-analysis. Inverse variance method and fixed effect model were used to combine the results of the primary studies. The heterogeneity between the results was assessed using Cochrane and I2 indices. Of 11,530 evidence identified during the primary search, three papers were found eligible for meta-analysis, including 7213 participants in the intervention groups and 7170 healthy controls. The efficacy (95% confidence interval) of HPV 6, 11, 16, 18 monovalent and quadrivalent vaccines against CIN 1, CIN 2, and CIN 3 were estimated as of 95% (88-98), 97% (85-99), and 95% (78-99), respectively. This study showed that L1 protein vaccines Cervarix and Gardasil are highly protective vaccines playing an effective role in the prevention of HPV 6, 11, 16, 18 which are responsible for CIN 1, CIN 2, and CIN 3.
Keywords: Cervical intraepithelial neoplasia; efficacy; human papillomavirus; meta-analysis; vaccine.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Cho HJ, Oh YK, Kim YB. Advances in human papilloma virus vaccines: A patent review. Expert Opin Ther Pat. 2011;21:295–309. - PubMed
-
- Ault KA, Giuliano AR, Edwards RP, Tamms G, Kim LL, Smith JF, et al. A phase I study to evaluate a human papillomavirus (HPV) type 18 L1 VLP vaccine. Vaccine. 2004;22:3004–7. - PubMed
-
- Park J, Sun D, Genest DR, Trivijitsilp P, Suh I, Crum CP. Coexistence of low and high grade squamous intraepithelial lesions of the cervix: Morphologic progression or multiple papillomaviruses? Gynecol Oncol. 1998;70:386–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources