Treatment of Neovascular Age-Related Macular Degeneration with Anti-VEGF Agents: Predictive Factors of Long-Term Visual Outcomes
- PMID: 28656102
- PMCID: PMC5471580
- DOI: 10.1155/2017/4263017
Treatment of Neovascular Age-Related Macular Degeneration with Anti-VEGF Agents: Predictive Factors of Long-Term Visual Outcomes
Abstract
Purpose: To evaluate the predictive factors of long-term visual outcomes in neovascular age-related macular degeneration (nAMD) treated with antivascular endothelial growth factor (anti-VEGF) agents.
Methods: Unicentric retrospective review of patients with nAMD treated with anti-VEGF agents. Visual outcomes, 12 and 60 months after diagnosis, were evaluated. In an attempt to identify predictive factors of visual outcomes, multiple variables (demographic and epidemiological characteristics, angiographic and tomographic features) were analyzed, at baseline and during follow-up.
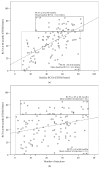
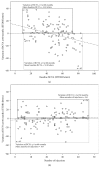
Results: One hundred and seventeen patients were included. In multivariate analysis, baseline best-corrected visual acuity was associated with all visual endpoints at 12 and 60 months. Additionally, age, gender, number of injections, and development of subretinal fibrosis during follow-up were also significant predictors of visual outcomes at 60 months.
Conclusions: Several factors can be useful in clinical practice as predictors of visual outcomes in response to anti-VEGF treatment of nAMD.
Figures

References
-
- Tsilimbaris M. K., López-Gálvez M. I., Gallego-Pinazo R., Margaron P., Lambrou G. N. Epidemiological and clinical baseline characteristics as predictive biomarkers of response to anti-VEGF treatment in patients with neovascular AMD. Journal of Ophthalmology. 2016;2016:13. doi: 10.1155/2016/4367631.4367631 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

