Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials
- PMID: 28656143
- PMCID: PMC5474547
- DOI: 10.1155/2017/4191670
Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials
Abstract
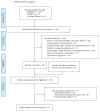
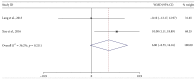
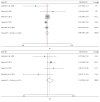
Cerebrolysin was reported to be effective in the neurological improvement of patients with acute ischemic stroke (AIS) in experimental models, while data from clinical trials were inconsistent. We performed a meta-analysis to explore the efficacy and safety of cerebrolysin for AIS. PubMed, EMBASE, and Cochrane Library were searched for randomized controlled trials, which intervened within 72 hours after the stroke onset. We investigated the efficacy and safety outcomes, respectively. Risk ratios and mean differences were pooled with fixed-effects model or random-effects model. Seven studies were identified, involving 1779 patients with AIS. The summary results failed to demonstrate significant superiority of cerebrolysin in the assessment of efficacy outcomes of mRS and BI. Similarly, administration of cerebrolysin had neutral effects on safety outcomes compared with placebo, including mortality and SAE. However, the number of included studies was small, especially in the analysis of efficacy outcomes, which might cause publication bias and inaccurate between-studies variance in the meta-analysis. Conclusively, although it seemed to be safe, routine use of cerebrolysin to improve the long-term rehabilitation after stroke could not be supported by available evidence.
Figures


References
-
- Murray C. J., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2197–2223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

