Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih
- PMID: 28656179
- PMCID: PMC5730045
- DOI: 10.1039/c7cc03580b
Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih
Abstract
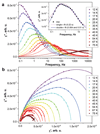
The Dy-Sc nitride clusterfullerene Dy2ScN@C80-Ih exhibits slow relaxation of magnetization up to 76 K. Above 60 K, thermally-activated relaxation proceeds via the fifth-excited Kramers doublet with the energy of 1735 ± 21 K, which is the highest barrier ever reported for dinuclear lanthanide single molecule magnets.
Figures




References
-
- Vieru V, Ungur L, Chibotaru LF. J Phys Chem Lett. 2013;4:3565–3569. - PubMed
- Cimpoesu F, Dragoe N, Ramanantoanina H, Urland W, Daul C. Phys Chem Chem Phys. 2014;16:11337–11348. - PubMed
- Zhang Y, Krylov D, Rosenkranz M, Schiemenz S, Popov AA. Chem Sci. 2015;6:2328–2341. - PMC - PubMed
- Singh MK, Rajaraman G. Chem Commun. 2016;52:14047–14050. - PubMed
-
- Sessoli R, Gatteschi D, Caneschi A, Novak MA. Nature. 1993;365:141–143.
- Habib F, Murugesu M. Chem Soc Rev. 2013;42:3278–3288. - PubMed
- Zhang P, Zhang L, Tang J. Dalton Trans. 2015;44:3923–3929. - PubMed
- Woodruff DN, Winpenny REP, Layfield RA. Chem Rev. 2013;113:5110–5148. - PubMed
- Luzon J, Sessoli R. Dalton Trans. 2012;41:13556–13567. - PubMed
- Sorace L, Benelli C, Gatteschi D. Chem Soc Rev. 2011;40:3092–3104. - PubMed
- Liddle ST, van Slageren J. Chem Soc Rev. 2015;44:6655–6669. - PubMed
- Rinehart JD, Long JR. Chem Sci. 2011;2:2078–2085.
-
- Gupta SK, Rajeshkumar T, Rajaraman G, Murugavel R. Chem Sci. 2016;7:5181–5191. - PMC - PubMed
- Chen Y-C, Liu J-L, Ungur L, Liu J, Li Q-W, Wang L-F, Ni Z-P, Chibotaru LF, Chen X-M, Tong M-L. J Am Chem Soc. 2016;138:2829–2837. - PubMed
- Ding Y-S, Chilton NF, Winpenny REP, Zheng Y-Z. Angew Chem, Int Ed. 2016;55:16071–16074. - PubMed
-
- Westerström R, Dreiser J, Piamonteze C, Muntwiler M, Weyeneth S, Brune H, Rusponi S, Nolting F, Popov A, Yang S, Dunsch L, Greber T. J Am Chem Soc. 2012;134:9840–9843. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

