Lentivirus‑mediated RIG‑I knockdown relieves cell proliferation inhibition, cell cycle arrest and apoptosis in ATRA‑induced NB4 cells via the AKT‑FOXO3A signaling pathway in vitro
- PMID: 28656276
- PMCID: PMC5547964
- DOI: 10.3892/mmr.2017.6858
Lentivirus‑mediated RIG‑I knockdown relieves cell proliferation inhibition, cell cycle arrest and apoptosis in ATRA‑induced NB4 cells via the AKT‑FOXO3A signaling pathway in vitro
Abstract
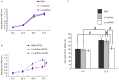
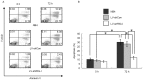
Retinoic acid inducible gene I (RIG‑I) is upregulated during all‑trans retinoic acid (ATRA)‑induced terminal granulocytic differentiation of NB4 acute promyelocytic leukemia (APL) cells. However, the function and mechanism of RIG‑I in NB4 cells remains to be fully elucidated. In the present study, lentivirus‑mediated RIG‑I‑knockdown was used to investigate the proliferation, cell cycle and apoptotic processes of ATRA‑induced NB4 cells in vitro using an MTT assay and flow cytometry, respectively. The roles of RIG‑I and the AKT‑FOXO3A signaling pathway were investigated using western blot analysis. The results showed that the ATRA‑induced expression of RIG‑I was specifically and effectively knocked down at the mRNA and protein levels by lentivirus mediated RIG‑I short hairpin RNA. In addition, silencing of RIG‑I reduced the ATRA‑induced inhibition of NB4 cell proliferation, cell cycle arrest and apoptosis. Further investigations indicated that with ATRA‑induced expression of RIG‑I, levels of phosphorylated (p)AKT‑Thr308 and pForkhead Box (FOX) O3A‑Thr32 were decreased, the expression levels of cell cycle arrest protein p27 and the apoptotic protein, tumor necrosis factor‑related apoptosis‑inducing ligand (TRAIL), directly transcribed by FOXO3A were increased. By contrast, following the knockdown of ATRA‑induced expression of RIG‑I, the levels of pAKT‑Thr308 and pFOXO3A‑Thr32 were increased, and the protein expression levels of p27 and TRAIL were decreased. Taken together, these results showed that the knockdown of RIG‑I reduced the inhibition of cell proliferation, cell cycle arrest and apoptosis in the ATRA‑induced NB4 cells via the AKT‑FOXO3A signaling pathway.
Figures


References
-
- Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH, Lanotte M, Waxman S, Chen SJ, et al. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

