99Tc-MDP-induced human osteoblast proliferation, differentiation and expression of osteoprotegerin
- PMID: 28656306
- PMCID: PMC5562099
- DOI: 10.3892/mmr.2017.6839
99Tc-MDP-induced human osteoblast proliferation, differentiation and expression of osteoprotegerin
Abstract
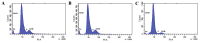
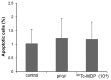
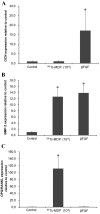
The aim of the present study was to examine the influence of technetium methylenediphosphonate (99Tc-MDP) on the proliferation and differentiation of human osteoblasts. Human iliac cancellous bone was isolated and cultured with either 99Tc‑MDP, β fibroblast growth factor (as a positive control) or medium only (as a negative control). Proliferation was assessed by direct cell counting, CCK‑8 assay and bromodeoxyuridine staining. The cell cycle and rate of apoptosis was assessed by propidium iodide staining and flow cytometry. Alkaline phosphatase (ALP) activity was assessed by the p‑nitrophenyl phosphate method and mineralized nodules were stained with Alizarin Red. Expression of osteocalcin (OCN) and bone morphogenetic protein‑2 (BMP‑2) was assessed by reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR), and expression levels of osteoprotegerin (OPG) and receptor activator of NF‑κB ligand (RANKL) were assessed by RT‑qPCR and ELISA. Isolated human osteoblasts stained positively for ALP and developed mineralized nodules. Treatment with 10‑5‑10‑10 M 99Tc‑MDP enhanced proliferation and 48 h incubation with 10‑8 M 99Tc‑MDP increased the proportion of cells in S‑phase, decreased the proportion in G0/G1 phase, and increased the cell proliferation index. The rate of apoptosis also increased, but the increase was not significant. Cells incubated with 10‑6‑10‑9 M 99Tc‑MDP for 3‑9 days exhibited increased ALP activity and mineralized nodule development. 10‑8 M 99Tc‑MDP increased BMP‑2 and OPG expression levels and OPG secretion, but OCN mRNA expression levels and RANKL secretion were not significantly altered at 72 h. 99Tc‑MDP treatment induced osteoblast proliferation and differentiation without affecting apoptosis. These findings provide proof of concept for the future use of 99Tc‑MDP in the treatment of bone‑destructive diseases.
Figures



Similar articles
-
Inhibitory effects of autologous γ-irradiated cell conditioned medium on osteoblasts in vitro.Mol Med Rep. 2015 Jul;12(1):273-80. doi: 10.3892/mmr.2015.3354. Epub 2015 Feb 16. Mol Med Rep. 2015. PMID: 25684548
-
Prolactin decreases the expression ratio of receptor activator of nuclear factor kappaB ligand/osteoprotegerin in human fetal osteoblast cells.Cell Biol Int. 2008 Sep;32(9):1126-35. doi: 10.1016/j.cellbi.2008.04.026. Epub 2008 May 9. Cell Biol Int. 2008. PMID: 18556224
-
Advanced glycation end products affect growth and function of osteoblasts.Clin Exp Rheumatol. 2011 Jul-Aug;29(4):650-60. Epub 2011 Aug 31. Clin Exp Rheumatol. 2011. PMID: 21906430
-
The effect of the alendronate on OPG/RANKL system in differentiated primary human osteoblasts.Endocrine. 2010 Feb;37(1):180-6. doi: 10.1007/s12020-009-9285-9. Epub 2009 Nov 24. Endocrine. 2010. PMID: 20963568
-
Effect of technetium-99 conjugated with methylene diphosphonate (99 Tc-MDP) on OPG/RANKL/RANK system in vitro.J Oral Pathol Med. 2019 Feb;48(2):129-135. doi: 10.1111/jop.12801. Epub 2018 Dec 9. J Oral Pathol Med. 2019. PMID: 30421571
Cited by
-
Caprylic acid (C8:0) promotes bone metastasis of prostate cancer by dysregulated adipo-osteogenic balance in bone marrow.Cancer Sci. 2020 Oct;111(10):3600-3612. doi: 10.1111/cas.14606. Epub 2020 Aug 28. Cancer Sci. 2020. PMID: 32770813 Free PMC article.
-
99Tc-Methylene Diphosphonate Treatment is Safe and Efficacious for Osteoporosis in Postmenopausal Differentiated Thyroid Cancer Patients Undergoing TSH Suppression: A Three-Center Non-Randomized Clinical Study.Cancer Manag Res. 2022 Mar 5;14:995-1005. doi: 10.2147/CMAR.S354471. eCollection 2022. Cancer Manag Res. 2022. PMID: 35283644 Free PMC article. Clinical Trial.
-
Puerarin promotes the viability and differentiation of MC3T3-E1 cells by enhancing LC3B-mediated autophagy through downregulation of miR-204.Exp Ther Med. 2020 Feb;19(2):883-890. doi: 10.3892/etm.2019.8291. Epub 2019 Dec 5. Exp Ther Med. 2020. PMID: 32010248 Free PMC article.
-
Bisphosphonate-incorporated coatings for orthopedic implants functionalization.Mater Today Bio. 2023 Jul 22;22:100737. doi: 10.1016/j.mtbio.2023.100737. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37576870 Free PMC article. Review.
-
16S rRNA Gene Sequencing of Gut Microbiota in Rheumatoid Arthritis Treated with 99Tc-MDP.Pharmgenomics Pers Med. 2024 May 23;17:237-249. doi: 10.2147/PGPM.S451065. eCollection 2024. Pharmgenomics Pers Med. 2024. PMID: 38807628 Free PMC article.
References
-
- Pockwinse SM, Stein JL, Lian JB, Stein GS. Developmental stage-specific cellular responses to vitamin D and glucocorticoids during differentiation of the osteoblast phenotype: Interrelationship of morphology and gene expression by in situ hybridization. Exp Cell Res. 1995;216:244–260. doi: 10.1006/excr.1995.1031. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

