On the structure and mechanism of two-pore channels
- PMID: 28656706
- PMCID: PMC5745306
- DOI: 10.1111/febs.14154
On the structure and mechanism of two-pore channels
Abstract
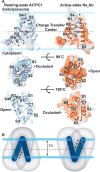
In eukaryotes, two-pore channels (TPC1-3) comprise a family of ion channels that regulate the conductance of Na+ and Ca2+ ions across cellular membranes. TPC1-3 form endolysosomal channels, but TPC3 can also function in the plasma membrane. TPC1/3 are voltage-gated channels, but TPC2 opens in response to binding endolysosome-specific lipid phosphatidylinositol-3,5-diphosphate (PI(3,5)P2 ). Filoviruses, such as Ebola, exploit TPC-mediated ion release as a means of escape from the endolysosome during infection. Antagonists that block TPC1/2 channel conductance abrogate filoviral infections. TPC1/2 form complexes with the mechanistic target of rapamycin complex 1 (mTORC1) at the endolysosomal surface that couple cellular metabolic state and cytosolic nutrient concentrations to the control of membrane potential and pH. We determined the X-ray structure of TPC1 from Arabidopsis thaliana (AtTPC1) to 2.87Å resolution-one of the two first reports of a TPC channel structure. Here, we summarize these findings and the implications that the structure may have for understanding endolysosomal control mechanisms and their role in human health.
Keywords: ion channels; lysosome; membrane protein; structure; transport.
© 2017 Federation of European Biochemical Societies.
Figures




References
-
- De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

