Adenosine Monophosphate Binding Stabilizes the KTN Domain of the Shewanella denitrificans Kef Potassium Efflux System
- PMID: 28656748
- PMCID: PMC5645763
- DOI: 10.1021/acs.biochem.7b00300
Adenosine Monophosphate Binding Stabilizes the KTN Domain of the Shewanella denitrificans Kef Potassium Efflux System
Abstract
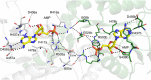
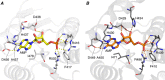
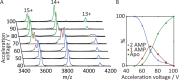
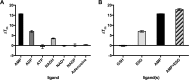
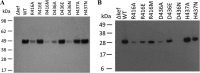
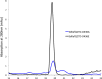
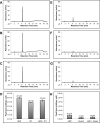
Ligand binding is one of the most fundamental properties of proteins. Ligand functions fall into three basic types: substrates, regulatory molecules, and cofactors essential to protein stability, reactivity, or enzyme-substrate complex formation. The regulation of potassium ion movement in bacteria is predominantly under the control of regulatory ligands that gate the relevant channels and transporters, which possess subunits or domains that contain Rossmann folds (RFs). Here we demonstrate that adenosine monophosphate (AMP) is bound to both RFs of the dimeric bacterial Kef potassium efflux system (Kef), where it plays a structural role. We conclude that AMP binds with high affinity, ensuring that the site is fully occupied at all times in the cell. Loss of the ability to bind AMP, we demonstrate, causes protein, and likely dimer, instability and consequent loss of function. Kef system function is regulated via the reversible binding of comparatively low-affinity glutathione-based ligands at the interface between the dimer subunits. We propose this interfacial binding site is itself stabilized, at least in part, by AMP binding.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Follmann M.; Becker M.; Ochrombel I.; Ott V.; Krämer R.; Marin K. (2009) Potassium transport in corynebacterium glutamicum is facilitated by the putative channel protein CglK, which is essential for pH homeostasis and growth at acidic pH. J. Bacteriol. 191, 2944–2952. 10.1128/JB.00074-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

