Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes
- PMID: 28656757
- PMCID: PMC5532695
- DOI: 10.1021/acs.chemrev.6b00816
Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes
Abstract
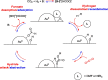
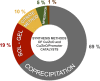
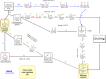
The recent advances in the development of heterogeneous catalysts and processes for the direct hydrogenation of CO2 to formate/formic acid, methanol, and dimethyl ether are thoroughly reviewed, with special emphasis on thermodynamics and catalyst design considerations. After introducing the main motivation for the development of such processes, we first summarize the most important aspects of CO2 capture and green routes to produce H2. Once the scene in terms of feedstocks is introduced, we carefully summarize the state of the art in the development of heterogeneous catalysts for these important hydrogenation reactions. Finally, in an attempt to give an order of magnitude regarding CO2 valorization, we critically assess economical aspects of the production of methanol and DME and outline future research and development directions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















References
-
- Princiotta F.Global Climate Change—The Technology Challenge; Springer: 2011; p 420.
-
- 2011 Technology Map of the European Strategic Energy Technology Plan (SET-Plan), 3rd ed.; European Commission, Joint Research Centre, Institute for Energy and Transport; © European Union: Luxembourg, 2011.
-
- The Global Status of CCS: 2011; The Global CCS Institute: Canberra, Australia, 2011.
-
- CCS EII Implementation Plan 2010–2012; Zero Emissions Platform: 2010.
-
- Rubin E. S.; Mantripragada H.; Marks A.; Versteeg P.; Kitchin J. The Outlook for Improved Carbon Capture Technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. 10.1016/j.pecs.2012.03.003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

