Formulation, Characterisation, and in Vitro Skin Diffusion of Nanostructured Lipid Carriers for Deoxyarbutin Compared to a Nanoemulsion and Conventional Cream
- PMID: 28656942
- PMCID: PMC5198023
- DOI: 10.3390/scipharm84040634
Formulation, Characterisation, and in Vitro Skin Diffusion of Nanostructured Lipid Carriers for Deoxyarbutin Compared to a Nanoemulsion and Conventional Cream
Abstract
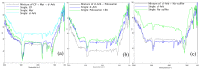
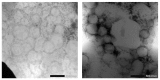
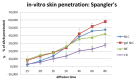
The long-term use of topical hydroquinone as an anti-hyperpigmentation treatment has well-known, unwanted effects. Deoxyarbutin (4-[(tetrahydro-2H-pyran-2-yl)oxy]phenol) is a relatively new tyrosinase inhibitor, with stronger inhibitory potency than hydroquinone, that exhibited decreased cytotoxicity against melanocytes and other cells. This study developed novel nanostructured lipid carriers (NLCs) for improved topical delivery of deoxyarbutin (dArb), leading to improved depigmenting efficacy. dArb is a hydrophobic substance, but it easily degrades in aqueous medium and is thermolabile. Screening and optimisation of the solid lipid, liquid lipid, surfactant, co-surfactant and production methods were performed to choose the optimum particle size and stability for NLCs. One percent dArb NLCs were obtained from a combination of cetyl palmitate (CP) and caprylic/capric tryglicerides (Myr) in 12% total lipids using poloxamer 188 (P-188) and polyethylene glycol (PEG) 400 as a surfactant and co-surfactant, respectively, with a particle diameter of approximately 500 nm and a polydispersity index (PI) <0.4. These NLCs were produced using the simple method of high-shear homogenisation (10,000 rpm, 5 minutes) and ultrasonication (3.5 min). The compatibility between the substances in the formula was evaluated using Fourier Transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). The morphology of the NLCs was observed using transmission electron microscopy (TEM). In vitro penetration of dArb NLCs was evaluated and compared to the nanoemulsion (NE) and conventional emulsion (CR) delivery methods across Spangler's membrane (SS). Delivery improvement was clearly observed, and after 8 h of application, dArb gel-NLCs showed the highest dArb penetration, followed by liquid NLCs, NE, and CR.
Keywords: cream; deoxyarbutin; in vitro diffusion; nanoemulsion; nanostructured lipid carriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chawla S., Kvalnes K., deLong M.A., Wickett R., Manga P., Boissy R.E. DeoxyArbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis. J. Drugs Dermatol. 2012;11:e28–e34. - PubMed
-
- Mihranyan A., Ferraz N., Strømme M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012;57:875–910. doi: 10.1016/j.pmatsci.2011.10.001. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
