Ketamine as an adjuvant to opioids for cancer pain
- PMID: 28657160
- PMCID: PMC6481583
- DOI: 10.1002/14651858.CD003351.pub3
Ketamine as an adjuvant to opioids for cancer pain
Abstract
Background: This is an update of a review first published in 2003 and updated in 2012.Ketamine is a commonly used anaesthetic agent, and in subanaesthetic doses is also given as an adjuvant to opioids for the treatment of refractory cancer pain, when opioids alone or in combination with appropriate adjuvant analgesics prove to be ineffective. Ketamine is known to have psychomimetic (including hallucinogenic), urological, and hepatic adverse effects.
Objectives: To determine the effectiveness and adverse effects of ketamine as an adjuvant to opioids for refractory cancer pain in adults.
Search methods: For this update, we searched MEDLINE (OVID) to December 2016. We searched CENTRAL (CRSO), Embase (OVID) and two clinical trial registries to January 2017.
Selection criteria: The intervention considered by this review was the addition of ketamine, given by any route of administration, in any dose, to pre-existing opioid treatment given by any route and in any dose, compared with placebo or active control. We included studies with a group size of at least 10 participants who completed the trial.
Data collection and analysis: Two review authors independently assessed the search results and performed 'Risk of bias' assessments. We aimed to extract data on patient-reported pain intensity, total opioid consumption over the study period; use of rescue medication; adverse events; measures of patient satisfaction/preference; function; and distress. We also assessed participant withdrawal (dropout) from trial. We assessed the quality of the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation).
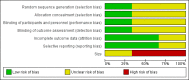
Main results: One new study (185 participants) was identified by the updated search and included in the review. We included a total of three studies in this update.Two small studies, both with cross-over design, with 20 and 10 participants respectively, were eligible for inclusion in the original review. One study with 20 participants examined the addition of intrathecal ketamine to intrathecal morphine, compared with intrathecal morphine alone. The second study with 10 participants examined the addition of intravenous ketamine bolus in two different doses to ongoing morphine therapy, compared with placebo. Both of these studies reported reduction in pain intensity and reduction in morphine requirements when ketamine was added to opioid for refractory cancer pain. The new study identified by the updated search had a parallel group design and 185 participants. This placebo-controlled study examined rapid titration of subcutaneous ketamine to high dose (500 mg) in participants who were using different opioids. There were no differences between groups for patient-reported pain intensity.Pooling of the data from the three included trials was not appropriate because of clinical heterogeneity.The study examining intrathecal drug administration reported no adverse events related to ketamine. In the study using intravenous bolus administration, ketamine caused hallucinations in four of 10 participants. In the rapid dose escalation/high-dose subcutaneous ketamine study, there was almost twice the incidence of adverse events in the ketamine group, compared to the placebo group, with the most common adverse events being needle site irritation and cognitive disturbance. Two serious adverse events (bradyarrhythmia and cardiac arrest) thought to be related to ketamine were also reported in this trial.For all three studies there was an unclear risk of bias overall. Using GRADE, we judged the quality of the evidence to be very low due to study limitations and imprecision due to the small number of participants in all comparisons.
Authors' conclusions: Current evidence is insufficient to assess the benefits and harms of ketamine as an adjuvant to opioids for the relief of refractory cancer pain. The evidence was of very low quality, meaning that it does not provide a reliable indication of the likely effect, and the likelihood that the effect will be substantially different is high. Rapid dose escalation of ketamine to high dose (500 mg) does not appear to have clinical benefit and may be associated with serious adverse events. More randomised controlled trials (RCTs) examining specific low-dose ketamine clinical regimens in current use are needed.
Conflict of interest statement
RFB: none known. RFB is a retired specialist pain physician who has worked with patients having acute, chronic or cancer pain, including palliative care patients.
CE: none known. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Phil Wiffen who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
EK: none known. EK is a specialist pain physician who has worked with patients having acute, chronic or cancer pain, including palliative care patients.
Figures
Update of
-
Ketamine as an adjuvant to opioids for cancer pain.Cochrane Database Syst Rev. 2012 Nov 14;11:CD003351. doi: 10.1002/14651858.CD003351.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jun 28;6:CD003351. doi: 10.1002/14651858.CD003351.pub3. PMID: 23152217 Updated.
References
References to studies included in this review
Hardy 2012 {published data only}
-
- Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, et al. Randomized, double‐blind, placebo‐controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. Journal of Clinical Oncology 2012;30(29):3611‐7. - PubMed
Mercadante 2000 {published data only}
-
- Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double‐blind, crossover, double‐dose study. Journal of Pain and Symptom Management 2000;4:246‐51. - PubMed
Yang 1996 {published data only}
-
- Yang CY, Wong CS, Chang JY. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Canadian Journal of Anaesthesia 1996;43(4):379‐83. - PubMed
References to studies excluded from this review
Currow 2011 {published data only}
-
- Currow DC, Hardy J, Sanderson C, Spruyt O, Eckermann S, Plummer J, et al. A randomised, double‐blind, placebo‐controlled, multi‐site study of subcutaneous ketamine in the management of cancer pain. European Journal of Cancer. 2011; Vol. 47:S152.
Ishizuka 2007 {published data only}
-
- Ishizuka P, Garcia JBS, Sakata RK, Issy AM, Mulich SL. Assessment of oral S(+) ketamine associated with morphine for the treatment of oncologic pain [Evaluación de la S(+) cetamina por vía oral asociada a la morfina en el tratamiento del dolor oncológico]. Revista Brasileira de Anestesiologia 2007;57(1):19‐31. - PubMed
Lauretti 1999a {published data only}
-
- Lauretti GR, Lima ICPR, Reis MP, Prado WA, Pereira NL. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology 1999;90:1528‐33. - PubMed
Lauretti 1999b {published data only}
-
- Lauretti GR, Gomes JMA, Reis MP, Pereira NL. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. Journal of Clinical Anaesthesia 1999;11(8):663‐8. - PubMed
Salas 2012 {published data only}
-
- Salas S, Frasca M, Planchet‐Barraud B, Burucoa B, Pascal M, Lapiana J‐M, et al. Ketamine analgesic effect by continuous intravenous infusion in refractory cancer pain: considerations about the clinical research in palliative care. Journal of Palliative Medicine 2012;15(3):287‐93. - PubMed
References to ongoing studies
NCT00484484 {published data only}
-
- Ketamine associated with opioids in refractory cancer pain treatment. Ongoing study May 2007. Completed September 2009.
NCT01207206 {published data only}
-
- Oral ketamine as an adjuvant to opioids for pain treatment in cancer patients. Ongoing study October 2010.
NCT01316744 {published data only}
-
- Ketamine hydrochloride and best pain management in treating cancer patients with neuropathic pain. Ongoing study April 2009.
NCT01326325 {published data only}
-
- Efficacy of low analgesic doses of ketamine associated with opioids in refractory cancer pain treatment. Ongoing study July 2011. Completed February 2013.
NCT02591017 {published data only}
-
- Comparison of oral morphine versus nasal ketamine spray with chitosan in cancer pain outpatients. Ongoing study February 2015.
Additional references
Afsharimani 2015
-
- Afsharimani B, Kindl K, Good P, Hardy J. Pharmacological options for the treatment of refractory cancer pain‐ what is the evidence?. Supportive Care in Cancer 2015;23(5):1473‐81. - PubMed
Are 2017
-
- Are M, McIntyre A, Reddy S. Global disparities in cancer pain management and palliative care. Journal of Surgical Oncology 2017;115(5):637‐41. - PubMed
Assouline 2016
-
- Assouline B, Tramèr MR, Kreienbühl L, Elia N. Benefit and harm of adding ketamine to an opioid in a patient‐controlled analgesia device for the control of postoperative pain: systematic review and meta‐analyses of randomized controlled trials with trial sequential analyses. Pain 2016;157(12):2854‐64. - PubMed
Bell 1999
-
- Bell RF. Low‐dose subcutaneous ketamine infusion and morphine tolerance. Pain 1999;83:101‐3. - PubMed
Bell 2006
Bell 2012a
-
- Bell RF. Ketamine for chronic noncancer pain: concerns regarding toxicity. Current Opinion in Supportive and Palliative Care 2012;6(2):183‐7. - PubMed
BNF 2012
-
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary. 63. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2012 (March).
Bredlau 2013
-
- Bredlau AL, Thakur R, Corones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Medicine 2013;14(10):1505‐17. - PubMed
Cancer Therapy Evaluation Program Version 3
-
- Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events V3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/....
Cherny 2015
-
- Cherny N, Fallon M, Kaasa S, Portenoy RK, Currow DC. Oxford Textbook of Palliative Medicine. 5th Edition. Oxford: Oxford University Press, 2015.
Dale 2012
-
- Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta‐analysis. Anesthesia and Analgesia 2012;115:934‐43. - PubMed
Dickenson 1994
-
- Dickenson AH. Neurophysiology of opioid poorly responsive pain. Cancer Surveys: Palliative Medicine: Problem Areas in Pain and Symptom Management 1994;21:5‐16. - PubMed
Dworkin 2008
-
- Dworkin RH, Turk DC, Wyrwich, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. - PubMed
Ebert 1997
-
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non‐competitive NMDA receptor antagonist in the rat brain and spinal cord. European Journal of Pharmacology 1997;333(1):91‐104. - PubMed
Edwards 2002
-
- Edwards SR, Minto CF, Mather LE. Concurrent ketamine and alfentanil administration:pharmacokinetic considerations. British Journal of Anaesthesia 2002;88(1):94‐100. - PubMed
Fallon 2013
-
- Fallon MT. Neuropathic pain in cancer. British Journal of Anaesthesia 2013;111(1):105‐11. - PubMed
Fine 1999
-
- Fine PG. Low‐dose ketamine in the management of opioid nonresponsive terminal cancer pain. Journal of Pain and Symptom Management 1999;17:296‐300. - PubMed
Fisher 2000
-
- Fisher K, Coderre TJ, Hagen NA. Targeting the N‐Methyl‐D‐Aspartate receptor for chronic pain management: preclinical animal studies, recent clinical experience and future research directions. Journal of Pain and Symptom Management 2000;20(5):358‐73. - PubMed
Fukuida 1981
-
- Fukuida E, Gocho C, Ito K. Pain in the terminal stage of cancer. IRYO Japanese Journal of National Medical Services 1981;35(6):584‐7.
Garry 1996
-
- Garry AC, Simpson KH. A difficult pain problem: Use of intrathecal ketamine. Pain Clinic 1996;9(3):335‐42.
GRADEpro GDT 2015 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro Guideline Development Tool [Software].. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org., 2015.
Grahame‐Smith 2002
-
- Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug herapy. 3rd Edition. Oxford: Oxford University Press, 2002.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.
Hijazi 2002
-
- Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N‐demethylation of ketamine in human liver microsomes. Drug Metabolism and Disposition 2002;30(7):853‐8. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kanamaru 1990
-
- Kanamaru T, Saeki S, Katsumata N, Mizuno K, Ogawa S, Suzuki H. Ketamine infusion for control of pain in patients with advanced cancer. Masui 1990;39(10):1368‐71. - PubMed
Karpinski 1997
-
- Karpinski N, Dunn J, Hansen L, Masliah E. Subpial vacuolar myelopathy after intrathecal ketamine: report of a case. Pain 1997;73(1):103‐5. - PubMed
Kharasch 1992
-
- Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology 1992;77(6):1201‐7. - PubMed
Klahr 1997
-
- Klahr M, Gomas JM, Larrouture A, Vinceneux P. Treatment of neurogenic pain in adult cancer patients. A retrospective study. Presse Medicale 1997;26(20):960‐3. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
Laskowski 2011
-
- Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Canadian Journal of Anesthesia 2011;58:911‐23. - PubMed
Laulin 2002
-
- Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl‐induced hyperalgesia and subsequent acute morphine tolerance. Anesthesia and Analgesia 2002;94(5):1263‐9. - PubMed
Leung 1986
-
- Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)‐6‐hydroxynorketamine. Journal of Medicinal Chemistry 1987;29(11):2396‐9. - PubMed
Lilius 2015
Lloyd‐Williams 2000
-
- Lloyd‐Williams M. Ketamine for cancer pain. Journal of Pain and Symptom Management 2000;19(2):79‐80. - PubMed
Lossignol 1992
-
- Lossignol D, Gil T, Rossi C, Sosnowski M, Obiols M, Vanmeerhaeghe B, et al. Cancer pain: A new concept. International Symposium on Supportive Care In Cancer; Bruges, Belgium. 8 ‐ 11 June, 1992:59.
Lossignol 1999
-
- Lossignol D, Obiols M, Body JJ. Ketamine and morphine in cancer pain [abstract]. 9th World Congress on Pain; Vienna, Austria. Seattle: International Association for the Study of Pain, 22 ‐ 27 August, 1999.
Mao 1995
-
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain 1995;62:259‐74. - PubMed
Mayer 1995
-
- Mayer DJ, Mao J, Price DD. The association of neuropathic pain, morphine tolerance and dependence, and the translocation of protein kinase C. NIDA‐Research Monographs 1995;147:269‐98. - PubMed
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998.
Mercadante 1995
-
- Mercadante S, Lodi F, Sapio M, Calligara M, Serretta R. Long‐term ketamine subcutaneous continuous‐infusion in neuropathic cancer pain. Journal of Pain and Symptom Management 1995;10(7):564‐8. - PubMed
Mitchell 1999
-
- Mitchell AC. Generalized hyperalgesia and allodynia following abrupt cessation of subcutaneous ketamine infusion. Palliative Medicine 1999;13(5):427‐8. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24.
Ogawa 1994
-
- Ogawa S, Kanamaru T, Noda K, Saeki S, Katsumata N, Kato J, et al. Intravenous microdrip infusion of ketamine in subanaesthetic doses for intractable terminal cancer pain. Pain Clinic 1994;7(2):125‐9.
Oshima 1990
-
- Oshima E, Tei K, Kayazawa H, Urabe N. Continuous subcutaneous injection of ketamine for cancer pain. Canadian Journal of Anesthesia 1990;37(3):385‐6. - PubMed
PaPaS 2012
-
- Pain, Palliative and Supportive Care Group. Authoring or assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group (AUREF). papas.cochrane.org/papas‐documents (accessed 6 April 2017).
Parada 1971
-
- Parada JF. Treatment of pain in cancer with ketamine hydrochloride. 13th Congreso Argentino de Anestesiologia, 1971 vol. 1; 101‐5. Buenos Aires, Oct 1971.
Peltoniemi 2016
-
- Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A review of clinical pharmacokinetics and pharmacodynamics in anaesthesia and pain therapy. Clinical Pharmacokinetics 2016;55(9):1059‐77. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre; The Cochrane Collaboration. Review Manager. Version 5.3.. Copenhagen: The Nordic Cochrane Centre; The Cochrane Collaboration, 2014.
Sawynok 2014
-
- Sawynok J. Topical and peripheral ketamine as an analgesic. Anesthesia and Analgesia 2014;119(1):170‐8. - PubMed
Smith 2000
-
- Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain 2000;86:119‐32. - PubMed
Sosnowski 1993
-
- Sosnowski M, Lossignol D, Fodderie L. Reversibility of opioid insensitive pain (abstract). World Congress on Pain. Seattle: IASP Publications, 1993:16.
Stannard 2005
-
- Stannard CF, Booth S. Churchill's Pocketbook of Pain. Second Edition. Edinburgh: Churchill Livingstone, 2005:384.
Stotz 1999
-
- Stotz M, Oehen HP, Gerber H. Histological findings after long‐term infusion of intrathecal ketamine for chronic pain: a case report. Journal of Pain and Symptom Management 1999;18(3):223‐8. - PubMed
Tarumi 2000
-
- Tarumi Y, Watanabe S, Bruera E, Ishitani K. High dose ketamine in the management of cancer‐related neuropathic pain. Journal of Pain and Symptom Management 2000;19(6):405‐7. - PubMed
Trujillo 1991
-
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK‐801. Science 1991;251(4989):85‐7. - PubMed
Twycross 2009
-
- Twycross R, Wilcock A, Toller CS. Symptom Management in Advanced Cancer. Fourth. Oxford: Palliativedrugs.com Ltd, 2009.
van den Beuken‐van Everdingen 2017
-
- Beuken‐van Everdingen MHJ, Graeff A, Jongen JLM, Dijkstra D, Mostovaya I, Vissers C, the national guideline working group "Diagnosis treatment of cancer pain". Pharmacological treatment of pain in cancer patients: the role of adjuvant analgesics, a systematic review. Pain Practice 2017;17(3):409‐19. - PubMed
Ventura 1993
-
- Ventura GJ, Blacklock DM. Intravenous ketamine HCL for treatment of intractable neuropathic cancer pain (INCP). Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1993;12:A1528.
Whizar‐Lugo 1987
-
- Whizar‐Lugo V, Cortez Gomez C. Epidural ketamine vs epidural morphine in severe cancer pain. Pain 1987;4 (Suppl):S142.
Willetts 1990
-
- Willetts J, Balster RL, Leander D. The behavioral pharmacology of NMDA receptor antagonists. Trends in Pharmacological Sciences 1990;11:423‐8. - PubMed
Wood 1997
-
- Wood T, Sloan R. Successful use of ketamine for central pain. Palliative Medicine 1997;11:57‐8. - PubMed
Woolf 1987
-
- Woolf TF, Adams JD. Biotransformation of ketamine, (Z)‐6‐hydroxyketamine, and (E)‐6‐hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica 1987;17(7):839‐47. - PubMed
Yanagihara 2001
-
- Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, et al. Involvement of CYP2B6 in n‐demethylation of ketamine in human liver microsomes. Drug Metabolism and Disposition 2001;29(6):887‐90. - PubMed
References to other published versions of this review
Bell 2003
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical