Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study
- PMID: 28657218
- PMCID: PMC5540716
- DOI: 10.3802/jgo.2017.28.e50
Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study
Abstract
Objective: To determine the clinical significance of the polymerase chain reaction (PCR)-reverse dot blot (RDB) human papillomavirus (HPV) genotyping assay in cervical cancer screening.
Methods: A total of 10,442 women attending the Fujian Provincial Maternity and Children's Health Hospital were evaluated using the liquid-based cytology (thinprep cytologic test [TCT]) and the PCR-RDB HPV test. Women with HPV infection and/or abnormal cytology were referred for colposcopy and biopsy. For HPV DNA sequencing, 120 specimens were randomly selected. Pathological diagnosis was used as the gold standard.
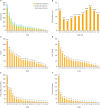
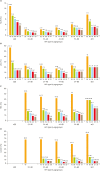
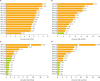
Results: Using the PCR-RDB HPV test, overall HPV prevalence was 20.57% (2,148/10,442) and that of high-risk (HR)-HPV infection was 18.68% (1,951/10,442). There was 99.2% concordance between HPV PCR-RDB testing and sequencing. In this studied population, the most common HR-HPV types were HPV-16, -52, -58, -18, -53, -33, and -51, rank from high to low. HPV-16, -18, -58, -59, and -33 were the top 5 prevalent genotypes in cervical cancer but HPV-16, -18, -59, -45, and -33 were the top 5 highest risk factors for cancer (odds ratio [OR]=34.964, 7.278, 6.728, 6.101, and 3.658; all p<0.05, respectively). Among 10,442 cases, 1,278 had abnormal cytology results, of which, the HR-HPV positivity rate was 83.02% (1,061/1,278). To screen for cervical cancer by PCR-RDB HPV testing, when using CIN2+, CIN3+, and cancer as observed endpoints, the sensitivity was 90.43%, 92.61%, and 94.78% and the negative predictive value (NPV) was 99.06%, 99.42%, and 99.78%, respectively. PCR-RDB HPV and TCT co-testing achieved the highest sensitivity and NPV.
Conclusion: For cervical cancer screening, the PCR-RDB HPV test can provide a reliable and sensitive clinical reference.
Keywords: Cancer Screening; Cell Biology; Genotype; Histology; Papillomaviridae.
Copyright © 2017. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon: International Agency for Research on Cancer; 2014.
-
- Meijer CJ, Snijders PJ, Castle PE. Clinical utility of HPV genotyping. Gynecol Oncol. 2006;103:12–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

