Anti-Cryptococcus Phenalenones and Cyclic Tetrapeptides from Auxarthron pseudauxarthron
- PMID: 28657331
- PMCID: PMC5629637
- DOI: 10.1021/acs.jnatprod.7b00341
Anti-Cryptococcus Phenalenones and Cyclic Tetrapeptides from Auxarthron pseudauxarthron
Abstract
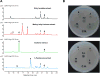
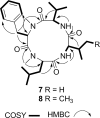
Auxarthrones A-E (1-5), five new phenalenones, and two new naturally occurring cyclic tetrapeptides, auxarthrides A (7) and B (8), were obtained from three different solvent extracts of cultures of the coprophilous fungus Auxarthron pseudauxarthron. Auxarthrones C (3) and E (5) possess an unusual 7a,8-dihydrocyclopenta[a]phenalene-7,9-dione ring system that has not been previously observed in natural products. Formation of 1-5 was found to be dependent on the solvent used for culture extraction. The structures of these new compounds were elucidated primarily by analysis of NMR and MS data. Auxarthrone A (1) was obtained as a mixture of chromatographically inseparable racemic diastereomers (1a and 1b) that cocrystallized, enabling confirmation of their structures by X-ray crystallography. The absolute configurations of 7 and 8 were assigned by analysis of their acid hydrolysates using Marfey's method. Compound 1 displayed moderate antifungal activity against Cryptococcus neoformans and Candida albicans, but did not affect human cancer cell lines.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
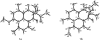

Similar articles
-
Antifungal cyclic peptides from Psammosilene tunicoides.J Nat Prod. 2010 Dec 27;73(12):1987-92. doi: 10.1021/np100363a. Epub 2010 Nov 11. J Nat Prod. 2010. PMID: 21070025
-
Phenalenone derivatives and the unusual tricyclic sesterterpene acid from the marine fungus Lophiostoma bipolare BCC25910.Phytochemistry. 2015 Dec;120:19-27. doi: 10.1016/j.phytochem.2015.11.003. Epub 2015 Nov 12. Phytochemistry. 2015. PMID: 26582262
-
Isolation and structural elucidation of proline-containing cyclopentapeptides from an endolichenic Xylaria sp.J Nat Prod. 2011 May 27;74(5):1303-8. doi: 10.1021/np100909y. Epub 2011 Mar 23. J Nat Prod. 2011. PMID: 21428374
-
Distribution, biosynthesis, and biological activity of phenylphenalenone-type compounds derived from the family of plants, Haemodoraceae.Nat Prod Rep. 2019 May 22;36(5):753-768. doi: 10.1039/c8np00067k. Nat Prod Rep. 2019. PMID: 30488050 Review.
-
Naturally occurring phenalenones and related compounds.Fortschr Chem Org Naturst. 1981;40:153-90. doi: 10.1007/978-3-7091-8611-4_4. Fortschr Chem Org Naturst. 1981. PMID: 7016694 Review. No abstract available.
Cited by
-
Natural Cyclic Peptides as an Attractive Modality for Therapeutics: A Mini Review.Molecules. 2018 Aug 20;23(8):2080. doi: 10.3390/molecules23082080. Molecules. 2018. PMID: 30127265 Free PMC article. Review.
-
Identification of cyclosporin C from Amphichorda felina using a Cryptococcus neoformans differential temperature sensitivity assay.Appl Microbiol Biotechnol. 2018 Mar;102(5):2337-2350. doi: 10.1007/s00253-018-8792-0. Epub 2018 Feb 2. Appl Microbiol Biotechnol. 2018. PMID: 29396588 Free PMC article.
-
A Decade of Antifungal Leads from Natural Products: 2010-2019.Pharmaceuticals (Basel). 2019 Dec 12;12(4):182. doi: 10.3390/ph12040182. Pharmaceuticals (Basel). 2019. PMID: 31842280 Free PMC article. Review.
-
Exploring the Activity of Fungal Phenalenone Derivatives as Potential CK2 Inhibitors Using Computational Methods.J Fungi (Basel). 2022 Apr 24;8(5):443. doi: 10.3390/jof8050443. J Fungi (Basel). 2022. PMID: 35628699 Free PMC article.
-
Sphaerostilbellins, New Antimicrobial Aminolipopeptide Peptaibiotics from Sphaerostilbella toxica.Biomolecules. 2020 Sep 26;10(10):1371. doi: 10.3390/biom10101371. Biomolecules. 2020. PMID: 32993102 Free PMC article.
References
-
- Harrison TS. AIDS. 2009;23:531–532. - PubMed
-
- Olsen L, Choffnes ER, Relman DA, Pray L. Fungal Diseases: An Emerging Threat to Human, Animal, and Plant Health: Workshop Summary. Washington DC: 2011. - PubMed
-
- Li Y, Yue Q, Krausert NM, An Z, Gloer JB, Bills GF. J. Nat. Prod. 2016;79:2357–2363. - PubMed
-
- Alvi KA, Rabenstein J. J. Ind. Microbiol. Biotechnol. 2004;31:11–15. - PubMed
-
- Nazir M, Harms H, Loef I, Kehraus S, El Maddah F, Arslan I, Rempel V, Muller CE, Konig GM. Planta Med. 2015;81:1141–1145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

