Role of Hypohalous Acids in Basement Membrane Homeostasis
- PMID: 28657332
- PMCID: PMC5647493
- DOI: 10.1089/ars.2017.7245
Role of Hypohalous Acids in Basement Membrane Homeostasis
Abstract
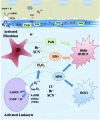
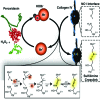
Significance: Basement membranes (BMs) are sheet-like structures of specialized extracellular matrix that underlie nearly all tissue cell layers including epithelial, endothelial, and muscle cells. BMs not only provide structural support but are also critical for the development, maintenance, and repair of organs. Animal heme peroxidases generate highly reactive hypohalous acids extracellularly and, therefore, target BMs for oxidative modification. Given the importance of BMs in tissue structure and function, hypohalous acid-mediated oxidative modifications of BM proteins represent a key mechanism in normal development and pathogenesis of disease. Recent Advances: Peroxidasin (PXDN), a BM-associated animal heme peroxidase, generates hypobromous acid (HOBr) to form sulfilimine cross-links within the collagen IV network of BM. These cross-links stabilize BM and are critical for animal tissue development. These findings highlight a paradoxical anabolic role for HOBr, which typically damages protein structure leading to dysfunction.
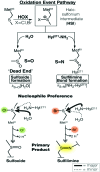
Critical issues: The molecular mechanism whereby PXDN uses HOBr as a reactive intermediate to cross-link collagen IV, yet avoid collateral damage to nearby BM proteins, remains unclear.
Future directions: The exact identification and functional impact of specific hypohalous acid-mediated modifications of BM proteins need to be addressed to connect these modifications to tissue development and pathogenesis of disease. As seen with the sulfilimine cross-link of collagen IV, hypohalous acid oxidative events may be beneficial in select situations rather than uniformly deleterious. Antioxid. Redox Signal. 27, 839-854.
Keywords: basement membrane; hypobromous acid; hypohalous acid; peroxidase; peroxidasin.
Figures



Similar articles
-
Peroxidasin-mediated bromine enrichment of basement membranes.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15827-15836. doi: 10.1073/pnas.2007749117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571911 Free PMC article.
-
Proprotein Convertase Processing Enhances Peroxidasin Activity to Reinforce Collagen IV.J Biol Chem. 2016 Nov 11;291(46):24009-24016. doi: 10.1074/jbc.M116.745935. Epub 2016 Oct 3. J Biol Chem. 2016. PMID: 27697841 Free PMC article.
-
The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F596-F602. doi: 10.1152/ajprenal.00096.2017. Epub 2017 Apr 19. Am J Physiol Renal Physiol. 2017. PMID: 28424209 Free PMC article.
-
The role of peroxidasin in solid cancer progression.Biochem Soc Trans. 2023 Oct 31;51(5):1881-1895. doi: 10.1042/BST20230018. Biochem Soc Trans. 2023. PMID: 37801286 Free PMC article. Review.
-
[Morphology of basement membrane and associated matrix proteins in normal and pathological tissues].Veroff Pathol. 1995;145:1-139. Veroff Pathol. 1995. PMID: 8638427 Review. German.
Cited by
-
Peroxidasin Inhibition by Phloroglucinol and Other Peroxidase Inhibitors.Antioxidants (Basel). 2023 Dec 21;13(1):23. doi: 10.3390/antiox13010023. Antioxidants (Basel). 2023. PMID: 38275643 Free PMC article.
-
Identification of brominated proteins in renal extracellular matrix: Potential interactions with peroxidasin.Biochem Biophys Res Commun. 2023 Nov 12;681:152-156. doi: 10.1016/j.bbrc.2023.09.063. Epub 2023 Sep 25. Biochem Biophys Res Commun. 2023. PMID: 37776746 Free PMC article.
-
Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer.Antioxid Redox Signal. 2017 Oct 20;27(12):874-911. doi: 10.1089/ars.2017.7140. Epub 2017 Sep 8. Antioxid Redox Signal. 2017. PMID: 28712304 Free PMC article.
-
Advances in the mechanism of small nucleolar RNA and its role in DNA damage response.Mil Med Res. 2024 Aug 8;11(1):53. doi: 10.1186/s40779-024-00553-4. Mil Med Res. 2024. PMID: 39118131 Free PMC article. Review.
-
Measuring peroxidasin activity in live cells using bromide addition for signal amplification.Redox Biol. 2022 Aug;54:102385. doi: 10.1016/j.redox.2022.102385. Epub 2022 Jun 30. Redox Biol. 2022. PMID: 35803124 Free PMC article.
References
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, and Yurchenco PD. A simplified laminin nomenclature. Matrix Biol 24: 326–332, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

