Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress
- PMID: 28657578
- PMCID: PMC5537786
- DOI: 10.3390/nu9070671
Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress
Abstract
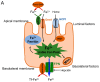
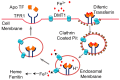
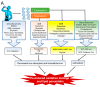
Oxidative stress is a common denominator in the pathogenesis of many chronic diseases. Therefore, antioxidants are often used to protect cells and tissues and reverse oxidative damage. It is well known that iron metabolism underlies the dynamic interplay between oxidative stress and antioxidants in many pathophysiological processes. Both iron deficiency and iron overload can affect redox state, and these conditions can be restored to physiological conditions using iron supplementation and iron chelation, respectively. Similarly, the addition of antioxidants to these treatment regimens has been suggested as a viable therapeutic approach for attenuating tissue damage induced by oxidative stress. Notably, many bioactive plant-derived compounds have been shown to regulate both iron metabolism and redox state, possibly through interactive mechanisms. This review summarizes our current understanding of these mechanisms and discusses compelling preclinical evidence that bioactive plant-derived compounds can be both safe and effective for managing both iron deficiency and iron overload conditions.
Keywords: antioxidants; ferroptosis; iron homeostasis; iron overload; oxidative stress; plant extracts.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

