Epidermal Growth Factor Receptor Signaling Enhances the Proinflammatory Effects of Staphylococcus aureus Gamma-Toxin on the Mucosa
- PMID: 28657583
- PMCID: PMC5535149
- DOI: 10.3390/toxins9070202
Epidermal Growth Factor Receptor Signaling Enhances the Proinflammatory Effects of Staphylococcus aureus Gamma-Toxin on the Mucosa
Abstract
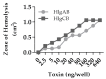
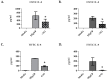
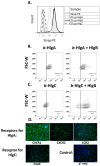
Staphylococcus aureus (S. aureus) produces many different exotoxins including the gamma-toxins, HlgAB and HlgCB. Gamma-toxins form pores in both leukocyte and erythrocyte membranes, resulting in cell lysis. The genes encoding gamma-toxins are present in most strains of S. aureus, and are commonly expressed in clinical isolates recovered from menstrual Toxic Shock Syndrome (mTSS) patients. This study set out to investigate the cytotoxic and proinflammatory effects of gamma-toxins on vaginal epithelial surfaces. We found that both HlgAB and HlgCB were cytotoxic to cultured human vaginal epithelial cells (HVECs) and induced cytokine production at sub-cytotoxic doses. Cytokine production induced by gamma-toxin treatment of HVECs was found to involve epidermal growth factor receptor (EGFR) signaling and mediated by shedding of EGFR ligands from the cell surface. The gamma-toxin subunits displayed differential binding to HVECs (HlgA 93%, HlgB 97% and HlgC 28%) with both components (HlgAB or HlgCB) required for maximum detectable binding and significant stimulation of cytokine production. In studies using full thickness ex vivo porcine vaginal mucosa, HlgAB or HlgCB stimulated a dose-dependent cytokine response, which was reduced significantly by inhibition of EGFR signaling. The effects of gamma-toxins on porcine vaginal tissue and cultured HVECs were validated using ex vivo human ectocervical tissue. Collectively, these studies have identified the EGFR-signaling pathway as a key component in gamma-toxin-induced proinflammatory changes at epithelial surfaces and highlight a potential therapeutic target to diminish toxigenic effects of S. aureus infections.
Keywords: Staphylococcus aureus; and mucosal immune response; epidermal growth factor receptor; gamma-toxin; menstrual toxic shock syndrome; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


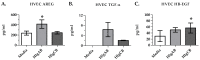



Similar articles
-
Use of porcine vaginal tissue ex-vivo to model environmental effects on vaginal mucosa to toxic shock syndrome toxin-1.Toxicol Appl Pharmacol. 2014 Jan 15;274(2):240-8. doi: 10.1016/j.taap.2013.11.021. Epub 2013 Dec 10. Toxicol Appl Pharmacol. 2014. PMID: 24333258
-
The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1.Infect Immun. 2005 Apr;73(4):2164-74. doi: 10.1128/IAI.73.4.2164-2174.2005. Infect Immun. 2005. PMID: 15784559 Free PMC article.
-
Epithelial proinflammatory response and curcumin-mediated protection from staphylococcal toxic shock syndrome toxin-1.PLoS One. 2012;7(3):e32813. doi: 10.1371/journal.pone.0032813. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431984 Free PMC article.
-
Ion channels and bacterial infection: the case of beta-barrel pore-forming protein toxins of Staphylococcus aureus.FEBS Lett. 2003 Sep 18;552(1):54-60. doi: 10.1016/s0014-5793(03)00850-0. FEBS Lett. 2003. PMID: 12972152 Review.
-
Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes.Biosci Biotechnol Biochem. 2004 May;68(5):981-1003. doi: 10.1271/bbb.68.981. Biosci Biotechnol Biochem. 2004. PMID: 15170101 Review.
Cited by
-
Host Cationic Antimicrobial Molecules Inhibit S. aureus Exotoxin Production.mSphere. 2023 Feb 21;8(1):e0057622. doi: 10.1128/msphere.00576-22. Epub 2023 Jan 4. mSphere. 2023. PMID: 36598227 Free PMC article.
-
Manipulation of Innate and Adaptive Immunity by Staphylococcal Superantigens.Pathogens. 2018 May 29;7(2):53. doi: 10.3390/pathogens7020053. Pathogens. 2018. PMID: 29843476 Free PMC article. Review.
-
Rapid reduction in Staphylococcus aureus in atopic dermatitis subjects following dupilumab treatment.J Allergy Clin Immunol. 2023 Nov;152(5):1179-1195. doi: 10.1016/j.jaci.2023.05.026. Epub 2023 Jun 13. J Allergy Clin Immunol. 2023. PMID: 37315812 Free PMC article. Clinical Trial.
-
The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections.Toxins (Basel). 2019 Jun 11;11(6):332. doi: 10.3390/toxins11060332. Toxins (Basel). 2019. PMID: 31212697 Free PMC article. Review.
-
Transfer of oral bacteria to the fetus during late gestation.Sci Rep. 2021 Jan 12;11(1):708. doi: 10.1038/s41598-020-80653-y. Sci Rep. 2021. PMID: 33436911 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

