Identification of Biologically Active Pyrimido[5,4-b]indoles That Prolong NF-κB Activation without Intrinsic Activity
- PMID: 28657707
- PMCID: PMC5841913
- DOI: 10.1021/acscombsci.7b00080
Identification of Biologically Active Pyrimido[5,4-b]indoles That Prolong NF-κB Activation without Intrinsic Activity
Abstract
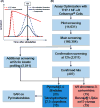
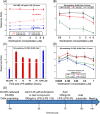
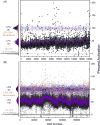
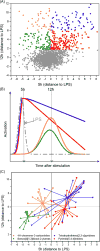
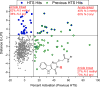
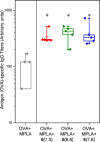
Most vaccine adjuvants directly stimulate and activate antigen presenting cells but do not sustain immunostimulation of these cells. A high throughput screening (HTS) strategy was designed to identify compounds that would sustain NF-κB activation by a stimulus from the Toll-like receptor (TLR)4 ligand, lipopolysaccharide (LPS). Several pilot studies optimized the parameters and conditions for a cell based NF-κB reporter assay in human monocytic THP-1 cells. The final assay evaluated prolongation of LPS induced NF-κB activation at 12 h. The dynamic range of the assay was confirmed in a pilot screen of 14 631 compounds and subsequently in a main extensive screen with 166 304 compounds. Hit compounds were identified using an enrichment strategy based on unsupervised chemoinformatic clustering, and also by a naı̈ve "Top X" approach. A total of 2011 compounds were then rescreened for levels of coactivation with LPS at 5 h and 12 h, which provided kinetic profiles. Of the 407 confirmed hits, compounds that showed correlation of the kinetic profiles with the structural similarities led to identification of four chemotypes: pyrimido[5,4-b]indoles, 4H-chromene-3-carbonitriles, benzo[d][1,3]dioxol-2-ylureas, and tetrahydrothieno[2,3-c]pyridines, which were segregated by 5 h and 12 h kinetic characteristics. Unlike the TLR4 agonistic pyrimidoindoles identified in previous studies, the revealed pyrimidoindoles in the present work did not intrinsically stimulate TLR4 nor induce NF-κB but rather prolonged NF-κB signaling induced by LPS. A 42-member combinatorial library was synthesized which led to identification of potent N3-alkyl substituted pyrimidoindoles that were not only active in vitro but also enhanced antibody responses in vivo when used as a coadjuvant. The novel HTS strategy led to identification of compounds that are intrinsically quiescent but functionally prolong stimulation by a TLR4 ligand and thereby potentiate vaccine efficacy.
Keywords: LPS; NF-κB; TLR4; adjuvant; pyrimidoindole.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, Bigham M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013;31(51):6122–8. - PubMed
-
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–608. - PubMed
-
- O’Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10(4):447–62. - PubMed
-
- Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: Protecting the vulnerable. Vaccine. 2016;34(52):6681–6690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

