Making waves in a photoactive polymer film
- PMID: 28658225
- PMCID: PMC5495175
- DOI: 10.1038/nature22987
Making waves in a photoactive polymer film
Abstract
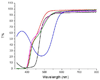
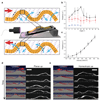
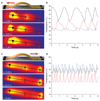
Oscillating materials that adapt their shapes in response to external stimuli are of interest for emerging applications in medicine and robotics. For example, liquid-crystal networks can be programmed to undergo stimulus-induced deformations in various geometries, including in response to light. Azobenzene molecules are often incorporated into liquid-crystal polymer films to make them photoresponsive; however, in most cases only the bending responses of these films have been studied, and relaxation after photo-isomerization is rather slow. Modifying the core or adding substituents to the azobenzene moiety can lead to marked changes in photophysical and photochemical properties, providing an opportunity to circumvent the use of a complex set-up that involves multiple light sources, lenses or mirrors. Here, by incorporating azobenzene derivatives with fast cis-to-trans thermal relaxation into liquid-crystal networks, we generate photoactive polymer films that exhibit continuous, directional, macroscopic mechanical waves under constant light illumination, with a feedback loop that is driven by self-shadowing. We explain the mechanism of wave generation using a theoretical model and numerical simulations, which show good qualitative agreement with our experiments. We also demonstrate the potential application of our photoactive films in light-driven locomotion and self-cleaning surfaces, and anticipate further applications in fields such as photomechanical energy harvesting and miniaturized transport.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Materials science: A light-fuelled wave machine.Nature. 2017 Jun 28;546(7660):604-606. doi: 10.1038/546604a. Nature. 2017. PMID: 28658241 No abstract available.
References
-
- Tabata O, Hirasawa H, Aoki S, Yoshida R, Kokufuta E. Ciliary motion actuator using self-oscillating gel. Sens Actuators Phys. 2002;95:234–238.
-
- Murase Y, Maeda S, Hashimoto S, Yoshida R. Design of a Mass Transport Surface Utilizing Peristaltic Motion of a Self-Oscillating Gel. Langmuir. 2009;25:483–489. - PubMed
-
- Maeda S, Hara Y, Yoshida R, Hashimoto S. Peristaltic Motion of Polymer Gels. Angew Chem Int Ed. 2008;47:6690–6693. - PubMed
-
- Maeda S, Hara Y, Sakai T, Yoshida R, Hashimoto S. Self-Walking Gel. Adv Mater. 2007;19:3480–3484.
-
- Martinez A, Smalyukh II. Light-driven dynamic Archimedes spirals and periodic oscillatory patterns of topological solitons in anisotropic soft matter. Opt Express. 2015;23:4591. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

