Antibiotics for neonates born through meconium-stained amniotic fluid
- PMID: 28658507
- PMCID: PMC6481483
- DOI: 10.1002/14651858.CD006183.pub2
Antibiotics for neonates born through meconium-stained amniotic fluid
Abstract
Background: Approximately 1 in 10 pregnancies is affected by meconium passage at delivery, which can result in meconium aspiration syndrome (MAS). MAS can cause respiratory complications and, very rarely, death. Antibiotics have been prescribed for neonates exposed to meconium in amniotic fluid, with the intention of preventing infection due to potential bacterial contaminants.
Objectives: We conducted this review to assess the efficacy and safety of antibiotics for:1. prevention of infection, morbidity, and mortality among infants born through meconium-stained amniotic fluid (MSAF) who are asymptomatic at birth; and2. prevention of infection, morbidity, and mortality among infants born through MSAF who have signs and symptoms compatible with meconium aspiration syndrome (MAS).
Search methods: We performed a literature search using the following databases: MEDLINE (1966 to July 2016); Embase (1980 to July 2016); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to July 2016); and the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 7) in the Cochrane Library. We also searched clinical trials databases, conference proceedings, and reference lists of retrieved articles.
Selection criteria: We included randomised and quasi-randomised controlled trials that compared antibiotics administered via any route versus placebo or no treatment for prevention of infection among neonates exposed to MSAF, or who developed MAS. We excluded cohort, case control, and any other non-randomised studies and applied no language restrictions. We included studies of term and preterm infants, and we included studies examining use of any antibacterial antibiotics. We included studies that reported on any outcomes of interest.
Data collection and analysis: We assessed the methodological quality of included trials by reviewing information provided in study reports and obtained by personal communication with study authors. We extracted data on relevant outcomes, estimated effect size, and reported values as risk ratios (RRs), risk differences (RDs), and mean differences (MDs), as appropriate. We conducted subgroup analyses for treatment of MAS and for prophylaxis (asymptomatic neonates exposed to meconium).
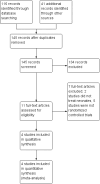
Main results: Four randomised controlled studies including a total of 695 participants were eligible for inclusion. Three studies evaluated neonates with MAS, and one study assessed asymptomatic neonates exposed to meconium in amniotic fluid. These studies exhibited varying degrees of methodological rigour: Two studies were at low risk of bias, and two were at unclear risk. We graded evidence derived from these studies as low quality. We downgraded overall evidence owing to the large number of participants lost to follow-up in one trial, the small sample sizes of all trials, and unclear methodological details provided for two trials.The primary outcome was risk of early- and late-onset neonatal sepsis. Antibiotics did not decrease the risk of sepsis in neonates with a diagnosis of MAS (RR 1.54, 95% confidence interval (CI) 0.27 to 8.96; RD 0.00, 95% CI -0.02 to 0.03; 445 participants, three studies; I² = 0%) nor in asymptomatic neonates exposed to meconium in amniotic fluid (RR 0.76, 95% CI 0.25 to 2.34; RD -0.01, 95% CI -0.07 to 0.04; 250 participants, one study; I² = 0%). Results show no significant differences in mortality or duration of stay in hospital between groups given antibiotics and control groups of symptomatic and asymptomatic neonates. One study in asymptomatic neonates reported a significant reduction in duration of mechanical ventilation for the control group compared with the antibiotic group (MD 0.26, 95% CI 0.15 to 0.37; 250 participants, one study; I² = 0%).
Authors' conclusions: Upon review of available evidence, we found no differences in infection rates following antibiotic treatment among neonates born through meconium-stained fluid and those with meconium aspiration syndrome. The overall quality of evidence is low owing to the small number of included studies. Well-controlled studies of adequate power are needed.
Conflict of interest statement
None.
Figures

























Update of
References
References to studies included in this review
Basu 2007 {published data only}
-
- Basu S, Kumar A, Bhatia BD. Role of antibiotics in meconium aspiration syndrome. Annals of Tropical Pediatrics 2007;27:107‐13. - PubMed
Goel 2015 {published data only}
-
- Goel A, Nangia S, Saili A, Garg A, Sharma S, Randhawa VS. Role of prophylactic antibiotics in neonates born through meconium‐stained amniotic fluid (MSAF) ‐ a randomized controlled trial. European Journal of Pediatrics 2015;174:237‐43. - PubMed
Lin 2005 {published data only}
-
- Lin HC, Su BH, Tsai CH, Lin TW, Yeh TF. Role of antibiotics in management of non‐ventilated cases of meconium aspiration syndrome without risk factors for infection. Biology of the Neonate 2005;87:51‐5. - PubMed
Shankar 1995 {published data only}
-
- Shankar V, Paul VK, Deorari AK, Singh M. Do neonates with meconium aspiration syndrome require antibiotics?. Indian Journal of Pediatrics 1995;62:327‐31. - PubMed
References to studies excluded from this review
Adair 1996 {published data only}
-
- Adair CD, Ernest JM, Sanchez‐Ramos L, Burrus DR, Boles ML, Veille JC. Meconium‐stained amniotic fluid‐associated infectious morbidity: a randomized, double‐blind trial of ampicillin‐sulbactam prophylaxis. Obstetrics and Gynecology 1996;88:216‐20. - PubMed
de Graff 1994 {published data only}
-
- Graaf MY, Verhagen E, Brand PL. Meconium‐containing amniotic fluid and what actions to take in newborn infants. Nederlands Tijdschrift voor Geneeskunde 1994;138(20):993‐5. - PubMed
Edwards 1999 {published data only}
Krishnan 1995 {published data only}
-
- Krishnan L, Nasruddin, Prabhakar P, Bhaskaranand N. Routine antibiotic cover for newborns intubated for aspirating meconium: is it necessary?. Indian Pediatrics 1995;32:529‐31. - PubMed
Pongmee 2015 {published data only}
-
- Pongmee P, Nagar G, Campbell S, Kumar M. Antibiotic administration for prevention or treatment of meconium aspiration syndrome in neonates: a systematic review. Jounral of Clinical Neonatology 2015;4:221‐6.
Siriwachirachai 2014 {published data only}
Vidyasagar 2013 {published data only}
-
- Vidyasagar D. The management of a neonate born with meconium stained amniotic fluid. Conference: 11th World Congress of Perinatal Medicine; Journal of Perinatal Medicine. 2013.
Additional references
Ahanya 2005
-
- Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstetrics and Gynecology Survey 2005;60:45‐56. - PubMed
Blot 1983
-
- Blot P, Milliez J, Breart G, Vige P, Nessmann C, Onufryk JP, et al. Fetal tachycardia and meconium staining: a sign of fetal infection. International Journal of Gynaecology and Obstetrics 1983;21:189‐94. - PubMed
Bortolucci 1990
-
- Bortolucci R, Seeliger HPR. Listeriosis. In: Remington JS, Klie JO editor(s). Infectious Diseases of the Fetus and Newborn Infant. 3rd Edition. Philadelphia: WB Saunders, 1990:812‐33.
Burgess 1996
-
- Burgess AM, Hutchins GM. Inflammation of the lungs, umbilical cord and placenta associated with meconium passage in utero. Review of 123 autopsied cases. Pathology, Research and Practice 1996;192:1121‐8. - PubMed
CDC 2004
-
- CDC guidelines. Campaign to prevent antimicrobial resistance in health care settings. 12 steps to prevent antimicrobial resistance among long term care residents. http://www.cdc.gov/drugresistance/healthcare/ltc/12steps_ltc.htm (accessed 5 June 2006).. Atlanta: Centers for Disease Control and Prevention, Atlanta: Centers for Disease Control and Prevention, 2004.
Cleary 1998
-
- Cleary GM, Wiswell TE. Meconium‐stained amniotic fluid and the meconium aspiration syndrome: an update. Pediatric Clinics of North America 1998;45:511‐29. - PubMed
Craig 2005
-
- Craig S, Lopez A, Hoskin D, Markham F. Meconium inhibits phagocytosis and stimulates respiratory burst in alveolar macrophages. Pediatric Research 2005;57:813‐8. - PubMed
Fanos 1999
-
- Fanos V, Citaldi L. Antibacterial‐induced nephrotoxicity in the newborn. Drug Safety 1999;20:245‐67. - PubMed
Florman 1969
-
- Florman AL, Teubner D. Enhancement of bacterial growth in amniotic fluid by meconium. Journal of Pediatrics 1969;74:111‐4. - PubMed
Gelfand 2004
-
- Gelfand SL, Fanaroff JM, Walsh MC. Controversies in the treatment of meconium aspiration syndrome. Clinics in Perinatology 2004;31:445‐52. - PubMed
Jiménez 2008
-
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile?. Research in Microbiology 2008;159:187–93. - PubMed
Lee 2004
-
- Lee JS, Stark AR. Meconium aspiration. In: Cloherty JP, Eichenwald EC, Stark AR editor(s). Manual of Neonatal Care. 5th Edition. Philadelphia: Lippincott Williams and Wilkins, 2004:402‐6.
Lembet 2003
-
- Lembet A, Gaddipati S, Holzman IR, Berkowitz RL, Bottone EJ. Meconium enhances the growth of perinatal bacterial pathogens. Mount Sinai Journal of Medicine 2003;70:126‐9. - PubMed
Madan 2012
Mazor 1995
-
- Mazor M, Furman B, Wiznitzer A, Shoham‐Vardi I, Cohen J, Ghezzi F. Maternal and perinatal outcome of patients with preterm labor and meconium‐stained amniotic fluid. Obstetrics and Gynecology 1995;86:830‐3. - PubMed
McCracken 1986
-
- McCracken GH Jr. Aminoglycoside toxicity in infants and children. The American Journal of Medicine 1986;80:172‐8. - PubMed
Mshvildadze 2010
Natarajan 2016
Rao 2001
-
- Rao S, Pavlova Z, Incerpi MH, Ramanathan R. Meconium‐stained amniotic fluid and neonatal morbidity in near‐term and term deliveries with acute histologic chorioamnionitis and/or funisitis. Journal of Perinatology 2001;21:537‐40. - PubMed
Romero 1991
-
- Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, et al. Meconium‐stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. American Journal of Obstetrics and Gynecology 1991;164:859‐62. - PubMed
Speer 1998
-
- Speer CP, Groneck P. Oxygen radicals, cytokines, adhesion molecules and lung injury in neonates. Seminars in Neonatology 1998;3:219‐28.
Tom‐Revzon 2004
-
- Tom‐Revzon C. Strategic use of antibiotics in the neonatal intensive care unit. The Journal of Perinatal and Neonatal Nursing 2004;18:241‐58. - PubMed
Usta 1995
-
- Usta IM, Mercer BM, Sibai BM. Risk factors for meconium aspiration syndrome. Obstetrics and Gynecology 1995;86:230‐4. - PubMed
Wan 2014
Warrier 2006
-
- Warrier I, Du W, Nataranjan G, Salari V, Aranda J. Patterns of drug utilization in a neonatal intensive care unit. The Journal of Clinical Pharmacology 2006;46(4):449‐55. - PubMed
Wiswell 1990
-
- Wiswell TE, Tuggle JM, Turner BS. Meconium aspiration syndrome: have we made a difference?. Pediatrics 1990;85:715‐21. - PubMed
Wiswell 1992
-
- Wiswell TE, Henley MA. Intratracheal suctioning, systemic infection, and the meconium aspiration syndrome. Pediatrics 1992;89:203‐6. - PubMed
Wiswell 1993
-
- Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatric Clinics of North America 1993;40:955‐81. - PubMed
Wiswell 2000
-
- Wiswell TE, Gannon CM, Jacob J, Goldsmith L, Szlyd E, Weiss K, et al. Delivery room management of the apparently vigorous meconium‐stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000;105:1‐7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

