Reverse pharmacogenomics: carbamazepine normalizes activation and attenuates thermal hyperexcitability of sensory neurons due to Nav 1.7 mutation I234T
- PMID: 28658526
- PMCID: PMC5980548
- DOI: 10.1111/bph.13935
Reverse pharmacogenomics: carbamazepine normalizes activation and attenuates thermal hyperexcitability of sensory neurons due to Nav 1.7 mutation I234T
Abstract
Background and purpose: Pharmacotherapy for pain currently involves trial and error. A previous study on inherited erythromelalgia (a genetic model of neuropathic pain due to mutations in the sodium channel, Nav 1.7) used genomics, structural modelling and biophysical and pharmacological analyses to guide pharmacotherapy and showed that carbamazepine normalizes voltage dependence of activation of the Nav 1.7-S241T mutant channel, reducing pain in patients carrying this mutation. However, whether this approach is applicable to other Nav channel mutants is still unknown.
Experimental approach: We used structural modelling, patch clamp and multi-electrode array (MEA) recording to assess the effects of carbamazepine on Nav 1.7-I234T mutant channels and on the firing of dorsal root ganglion (DRG) sensory neurons expressing these mutant channels.
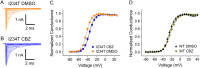
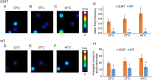
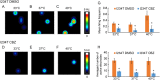
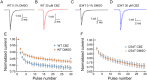
Key results: In a reverse engineering approach, structural modelling showed that the I234T mutation is located in atomic proximity to the carbamazepine-responsive S241T mutation and that activation of Nav 1.7-I234T mutant channels, from patients who are known to respond to carbamazepine, is partly normalized with a clinically relevant concentration (30 μM) of carbamazepine. There was significantly higher firing in intact sensory neurons expressing Nav 1.7-I234T channels, compared with neurons expressing the normal channels (Nav 1.7-WT). Pre-incubation with 30 μM carbamazepine also significantly reduced the firing of intact DRG sensory neurons expressing Nav 1.7-I234T channels. Although the expected use-dependent inhibition of Nav 1.7-WT channels by carbamazepine was confirmed, carbamazepine did not enhance use-dependent inhibition of Nav 1.7-I234T mutant channels.
Conclusion and implications: These results support the utility of a pharmacogenomic approach to treatment of pain in patients carrying sodium channel variants.
Linked articles: This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc.
© 2017 The British Pharmacological Society.
Figures


Similar articles
-
Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel.Nat Commun. 2012;3:1186. doi: 10.1038/ncomms2184. Nat Commun. 2012. PMID: 23149731 Free PMC article.
-
Pharmacotherapy for Pain in a Family With Inherited Erythromelalgia Guided by Genomic Analysis and Functional Profiling.JAMA Neurol. 2016 Jun 1;73(6):659-67. doi: 10.1001/jamaneurol.2016.0389. JAMA Neurol. 2016. PMID: 27088781
-
Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article.
-
Structure-based assessment of disease-related mutations in human voltage-gated sodium channels.Protein Cell. 2017 Jun;8(6):401-438. doi: 10.1007/s13238-017-0372-z. Epub 2017 Feb 1. Protein Cell. 2017. PMID: 28150151 Free PMC article. Review.
-
Fifteen years of NaV 1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy?Br J Pharmacol. 2022 Jul;179(14):3592-3611. doi: 10.1111/bph.15327. Epub 2020 Dec 18. Br J Pharmacol. 2022. PMID: 33206998 Review.
Cited by
-
The Novel Activity of Carbamazepine as an Activation Modulator Extends from NaV1.7 Mutations to the NaV1.8-S242T Mutant Channel from a Patient with Painful Diabetic Neuropathy.Mol Pharmacol. 2018 Nov;94(5):1256-1269. doi: 10.1124/mol.118.113076. Epub 2018 Aug 22. Mol Pharmacol. 2018. PMID: 30135145 Free PMC article.
-
Resilience to Pain: A Peripheral Component Identified Using Induced Pluripotent Stem Cells and Dynamic Clamp.J Neurosci. 2019 Jan 16;39(3):382-392. doi: 10.1523/JNEUROSCI.2433-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459225 Free PMC article.
-
Inhibition of intracellular proton-sensitive Ca2+-permeable TRPV3 channels protects against ischemic brain injury.Acta Pharm Sin B. 2022 May;12(5):2330-2347. doi: 10.1016/j.apsb.2022.01.001. Epub 2022 Jan 7. Acta Pharm Sin B. 2022. PMID: 35646518 Free PMC article.
-
Using stratified medicine to understand, diagnose, and treat neuropathic pain.Pain. 2018 Sep;159 Suppl 1(Suppl 1):S31-S42. doi: 10.1097/j.pain.0000000000001301. Pain. 2018. PMID: 30113945 Free PMC article. Review. No abstract available.
-
Deficiency of anti-inflammatory cytokine IL-4 leads to neural hyperexcitability and aggravates cerebral ischemia-reperfusion injury.Acta Pharm Sin B. 2020 Sep;10(9):1634-1645. doi: 10.1016/j.apsb.2020.05.002. Epub 2020 May 20. Acta Pharm Sin B. 2020. PMID: 33088684 Free PMC article.
References
-
- Ahn HS, Dib‐Hajj SD, Cox JJ, Tyrrell L, Elmslie FV, Clarke AA et al. (2010). A new Nav1.7 sodium channel mutation I234T in a child with severe pain. Eur J Pain 14: 944–950. - PubMed
-
- Atkins JF, Wills NM, Loughran G, Wu CY, Parsawar K, Ryan MD et al. (2007). A case for “StopGo”: reprogramming translation to augment codon meaning of GGN by promoting unconventional termination (Stop) after addition of glycine and then allowing continued translation (Go). RNA 13: 803–810. - PMC - PubMed
-
- Breton H, Cociglio M, Bressolle F, Peyriere H, Blayac JP, Hillaire‐Buys D (2005). Liquid chromatography‐electrospray mass spectrometry determination of carbamazepine, oxcarbazepine and eight of their metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 828: 80–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

