Peripheral Sensory Deprivation Restores Critical-Period-like Plasticity to Adult Somatosensory Thalamocortical Inputs
- PMID: 28658619
- PMCID: PMC7443048
- DOI: 10.1016/j.celrep.2017.06.018
Peripheral Sensory Deprivation Restores Critical-Period-like Plasticity to Adult Somatosensory Thalamocortical Inputs
Abstract
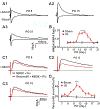
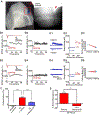
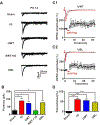
Recent work has shown that thalamocortical (TC) inputs can be plastic after the developmental critical period has closed, but the mechanism that enables re-establishment of plasticity is unclear. Here, we find that long-term potentiation (LTP) at TC inputs is transiently restored in spared barrel cortex following either a unilateral infra-orbital nerve (ION) lesion, unilateral whisker trimming, or unilateral ablation of the rodent barrel cortex. Restoration of LTP is associated with increased potency at TC input and reactivates anatomical map plasticity induced by whisker follicle ablation. The reactivation of TC LTP is accompanied by reappearance of silent synapses. Both LTP and silent synapse formation are preceded by transient re-expression of synaptic GluN2B-containing N-methyl-D-aspartate (NMDA) receptors, which are required for the reappearance of TC plasticity. These results clearly demonstrate that peripheral sensory deprivation reactivates synaptic plasticity in the mature layer 4 barrel cortex with features similar to the developmental critical period.
Keywords: NMDA receptor; adult barrel cortex; experience-dependent plasticity; glutamate; long-term potentiation; silent synapses; synaptic plasticity.
Published by Elsevier Inc.
Figures




References
-
- Agmon A, and Connors BW (1991). Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379. - PubMed
-
- Barth AL, and Malenka RC (2001). NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4, 235–236. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

