The Role of Fibroblasts in Pancreatic Cancer: Extracellular Matrix Versus Paracrine Factors
- PMID: 28658650
- PMCID: PMC5487255
- DOI: 10.1016/j.tranon.2017.04.009
The Role of Fibroblasts in Pancreatic Cancer: Extracellular Matrix Versus Paracrine Factors
Abstract
Background and aim: Desmoplasia is a characteristic feature and a suspected mechanism of tumor progression in pancreatic ductal adenocarcinoma (PDAC). Main constituents of the stroma involve cancer-associated fibroblasts (CAFs) and extracellular matrix (ECM). The aim of this study was to dissect the interaction of CAFs, ECM, and PDAC cells in both an in vitro setting and a large-scale clinical cohort study.
Methods and material: Patients operated for PDAC were identified from our prospectively maintained clinical database. A standard pathology protocol was applied for pancreatoduodenectomy specimens also assessing CAF activation as either CAF grade 0 or CAF grade +. Interaction between a spectrum of pancreatic cancer cell lines (PCCs) and mouse embryonic fibroblasts (NIH 3T3) was assessed in a conditioned medium experimental setup.
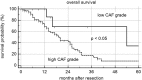
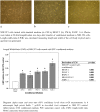
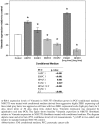
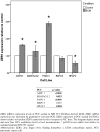
Results: One hundred eleven patients operated for PDAC from 2001 to 2011 were identified. Univariate analysis disclosed CAF grade + (P = .030), positive M status (P < .001), and lymph node ratio (LNR) > 0.1 (P = .045) to impair overall survival. Independent prognostic factors were CAF grade (P = .050) and positive M status (P = .002). CAF grade correlated with N status (CC = 0.206, P = .030), LNR (CC = 0.187, P = .049), tumor size (CC = -0.275, P = .003), and M status (CC = 0.190, P = .045). In the in vitro setting, paracrine effects of pancreatic cancer cell resulted in morphological activation of fibroblasts and tumor cell differentiation-dependent increase of fibroblast growth. Paracrine effects of poorly differentiated PCCs led to an upregulation of Vimentin in NIH 3T3 fibroblasts. Paracrine effects of fibroblasts on their part promoted cancer cell motility in all PCCs. As the second stromal component, fibroblast-derived ECM resulted in significantly decreased proliferation depending on density and led to upregulation of ZEB1 in poorly differentiated PCCs.
Conclusion: In PDAC patients, positive CAF grading was identified as a negative prognostic parameter correlating with positive N status, high LNR, positive M status, and smaller tumor size. Whereas bilateral interaction of PCCs and CAFs promotes tumor progression, ECM poses PCC growth restrictions. In summary, our study discloses differential effects of stromal components and may help to interpret heterogeneous results of former studies.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Lillemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: state-of-the-art care. CA Cancer J Clin. 2000;50(4):241–268. - PubMed
-
- Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg. 1995;82:111–115. - PubMed
-
- Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22:699–701. - PubMed
-
- Preca BT, Bajdak K, Mock K, Sundararajan V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz S. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer. 2015;137:2566–2577. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

