The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson's disease
- PMID: 28659169
- PMCID: PMC5490188
- DOI: 10.1186/s40478-017-0456-2
The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson's disease
Erratum in
-
Correction to: The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson's disease.Acta Neuropathol Commun. 2021 Sep 29;9(1):161. doi: 10.1186/s40478-021-01258-8. Acta Neuropathol Commun. 2021. PMID: 34588001 Free PMC article. No abstract available.
Abstract
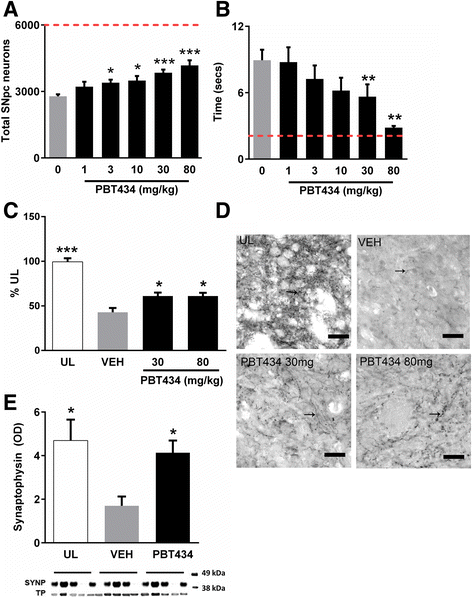
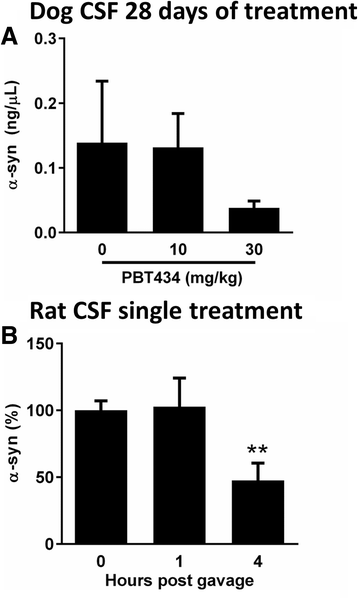
Elevated iron in the SNpc may play a key role in Parkinson's disease (PD) neurodegeneration since drug candidates with high iron affinity rescue PD animal models, and one candidate, deferirpone, has shown efficacy recently in a phase two clinical trial. However, strong iron chelators may perturb essential iron metabolism, and it is not yet known whether the damage associated with iron is mediated by a tightly bound (eg ferritin) or lower-affinity, labile, iron pool. Here we report the preclinical characterization of PBT434, a novel quinazolinone compound bearing a moderate affinity metal-binding motif, which is in development for Parkinsonian conditions. In vitro, PBT434 was far less potent than deferiprone or deferoxamine at lowering cellular iron levels, yet was found to inhibit iron-mediated redox activity and iron-mediated aggregation of α-synuclein, a protein that aggregates in the neuropathology. In vivo, PBT434 did not deplete tissue iron stores in normal rodents, yet prevented loss of substantia nigra pars compacta neurons (SNpc), lowered nigral α-synuclein accumulation, and rescued motor performance in mice exposed to the Parkinsonian toxins 6-OHDA and MPTP, and in a transgenic animal model (hA53T α-synuclein) of PD. These improvements were associated with reduced markers of oxidative damage, and increased levels of ferroportin (an iron exporter) and DJ-1. We conclude that compounds designed to target a pool of pathological iron that is not held in high-affinity complexes in the tissue can maintain the survival of SNpc neurons and could be disease-modifying in PD.
Keywords: Chelation; Drug development; Neuroprotection; Oxidative stress; Synucleinopathy.
Figures





References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. - DOI - PubMed
-
- Adlard PA, Bica L, White AR, Nurjono M, Filiz G, Crouch PJ, Donnelly PS, Cappai R, Finkelstein DI, Bush AI. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease. PLoS One. 2011;6:e17669. doi: 10.1371/journal.pone.0017669. - DOI - PMC - PubMed
-
- Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978;253:1930–1937. - PubMed
-
- Ayton S, Lei P, Hare DJ, Duce JA, George JL, Adlard PA, McLean C, Rogers JT, Cherny RA, Finkelstein DI, et al. Parkinson’s disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci. 2015;35:3591–3597. doi: 10.1523/JNEUROSCI.3439-14.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

