Overload-mediated skeletal muscle hypertrophy is not impaired by loss of myofiber STAT3
- PMID: 28659288
- PMCID: PMC5625092
- DOI: 10.1152/ajpcell.00100.2017
Overload-mediated skeletal muscle hypertrophy is not impaired by loss of myofiber STAT3
Abstract
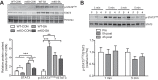
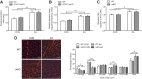
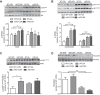
Although the signal pathways mediating muscle protein synthesis and degradation are well characterized, the transcriptional processes modulating skeletal muscle mass and adaptive growth are poorly understood. Recently, studies in mouse models of muscle wasting or acutely exercised human muscle have suggested a potential role for the transcription factor signal transducer and activator of transcription 3 (STAT3), in adaptive growth. Hence, in the present study we sought to define the contribution of STAT3 to skeletal muscle adaptive growth. In contrast to previous work, two different resistance exercise protocols did not change STAT3 phosphorylation in human skeletal muscle. To directly address the role of STAT3 in load-induced (i.e., adaptive) growth, we studied the anabolic effects of 14 days of synergist ablation (SA) in skeletal muscle-specific STAT3 knockout (mKO) mice and their floxed, wild-type (WT) littermates. Plantaris muscle weight and fiber area in the nonoperated leg (control; CON) was comparable between genotypes. As expected, SA significantly increased plantaris weight, muscle fiber cross-sectional area, and anabolic signaling in WT mice, although interestingly, this induction was not impaired in STAT3 mKO mice. Collectively, these data demonstrate that STAT3 is not required for overload-mediated hypertrophy in mouse skeletal muscle.
Keywords: STAT3; hypertrophy; resistance exercise; skeletal muscle.
Copyright © 2017 the American Physiological Society.
Figures
Comment in
-
Defining the STATus quo in muscle hypertrophy. Focus on "Overload-mediated skeletal muscle hypertrophy is not impaired by loss of myofiber STAT3".Am J Physiol Cell Physiol. 2017 Sep 1;313(3):C255-C256. doi: 10.1152/ajpcell.00165.2017. Epub 2017 Aug 2. Am J Physiol Cell Physiol. 2017. PMID: 28768642 No abstract available.
References
-
- Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303: E410–E421, 2012. doi:10.1152/ajpendo.00039.2012. - DOI - PMC - PubMed
-
- Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 111: 135–142, 2011. doi:10.1152/japplphysiol.01408.2010. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

