Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants
- PMID: 28659297
- PMCID: PMC5573022
- DOI: 10.3945/ajcn.117.152967
Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants
Abstract
Background: The pathophysiology of necrotizing enterocolitis (NEC) remains poorly understood.Objective: We assessed the relation between feeding strategies, intestinal microbiota composition, and the development of NEC.Design: We performed a prospective nationwide population-based study, EPIPAGE 2 (Etude Epidémiologique sur les Petits Ages Gestationnels), including preterm infants born at <32 wk of gestation in France in 2011. From individual characteristics observed during the first week of life, we calculated a propensity score for the risk of NEC (Bell's stage 2 or 3) after day 7 of life. We analyzed the relation between neonatal intensive care unit (NICU) strategies concerning the rate of progression of enteral feeding, the direct-breastfeeding policy, and the onset of NEC using general linear mixed models to account for clustering by the NICU. An ancillary propensity-matched case-control study, EPIFLORE (Etude Epidémiologique de la flore), in 20 of the 64 NICUs, analyzed the intestinal microbiota by culture and 16S ribosomal RNA gene sequencing.Results: Among the 3161 enrolled preterm infants, 106 (3.4%; 95% CI: 2.8%, 4.0%) developed NEC. Individual characteristics were significantly associated with NEC. Slower and intermediate rates of progression of enteral feeding strategies were associated with a higher risk of NEC, with an adjusted OR of 2.3 (95% CI: 1.2, 4.5; P = 0.01) and 2.0 (95% CI: 1.1, 3.5; P = 0.02), respectively. Less favorable and intermediate direct-breastfeeding policies were associated with higher NEC risk as well, with an adjusted OR of 2.5 (95% CI: 1.1, 5.8; P = 0.03) and 2.3 (95% CI: 1.1, 4.8; P = 0.02), respectively. Microbiota analysis performed in 16 cases and 78 controls showed an association between Clostridium neonatale and Staphylococcus aureus with NEC (P = 0.001 and P = 0.002).Conclusions: A slow rate of progression of enteral feeding and a less favorable direct-breastfeeding policy are associated with an increased risk of developing NEC. For a given level of risk assessed by propensity score, colonization by C. neonatale and/or S. aureus is significantly associated with NEC. This trial (EPIFLORE study) was registered at clinicaltrials.gov as NCT01127698.
Keywords: breastfeeding; clostridia; necrotizing enterocolitis; preterm infant; speed of increasing enteral nutrition.
© 2017 American Society for Nutrition.
Figures

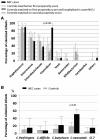
Comment in
-
Rapid standardized enteral feeding strategy in preterm infants: is it safe?Am J Clin Nutr. 2017 Sep;106(3):713-714. doi: 10.3945/ajcn.117.164186. Epub 2017 Aug 9. Am J Clin Nutr. 2017. PMID: 28793988 Free PMC article. No abstract available.
References
-
- Lin PW, Stoll BJ.. Necrotising enterocolitis. Lancet 2006;368:1271–83. - PubMed
-
- Neu J.. Necrotizing enterocolitis: the mystery goes on. Neonatology 2014;106:289–95. - PubMed
-
- AlFaleh K, Anabrees J.. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;4:CD005496. - PubMed
-
- Deshpande G, Rao S, Patole S.. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007;369:1614–20. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

