Identification of an Atypical Enzootic Bovine Leukosis in Japan by Using a Novel Classification of Bovine Leukemia Based on Immunophenotypic Analysis
- PMID: 28659325
- PMCID: PMC5585698
- DOI: 10.1128/CVI.00067-17
Identification of an Atypical Enzootic Bovine Leukosis in Japan by Using a Novel Classification of Bovine Leukemia Based on Immunophenotypic Analysis
Erratum in
-
Correction for Nishimori et al., “Identification of an Atypical Enzootic Bovine Leukosis in Japan by Using a Novel Classification of Bovine Leukemia Based on Immunophenotypic Analysis”.Clin Vaccine Immunol. 2018 Apr 17;24(12):e00001-18. doi: 10.1128/CVI.00001-18. Print 2017 Dec. Clin Vaccine Immunol. 2018. PMID: 29741849 No abstract available.
Abstract
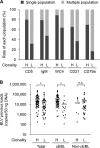
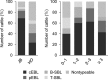
Bovine leukemia is classified into two types: enzootic bovine leukosis (EBL) and sporadic bovine leukosis (SBL). EBL is caused by infection with bovine leukemia virus (BLV), which induces persistent lymphocytosis and B-cell lymphoma in cattle after a long latent period. Although it has been demonstrated that BLV-associated lymphoma occurs predominantly in adult cattle of >3 to 5 years, suspicious cases of EBL onset in juvenile cattle were recently reported in Japan. To investigate the current status of bovine leukemia in Japan, we performed immunophenotypic analysis of samples from 50 cattle that were clinically diagnosed as having bovine leukemia. We classified the samples into five groups on the basis of the analysis and found two different types of EBL: classic EBL (cEBL), which has the familiar phenotype commonly known as EBL, and polyclonal EBL (pEBL), which exhibited neoplastic proliferation of polyclonal B cells. Moreover, there were several atypical EBL cases even in cEBL, including an early onset of EBL in juvenile cattle. A comparison of the cell marker expressions among cEBL, pEBL, and B-cell-type SBL (B-SBL) revealed characteristic patterns in B-cell leukemia, and these patterns could be clearly differentiated from those of healthy phenotypes, whereas it was difficult to discriminate between cEBL, pEBL, and B-SBL only by the expression patterns of cell markers. This study identified novel characteristics of bovine leukemia that should contribute to a better understanding of the mechanism underlying tumor development in BLV infection.
Keywords: bovine leukemia; early onset EBL; expression patterns of B-cell markers; immunophenotyping.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Clone Dynamics and Its Application for the Diagnosis of Enzootic Bovine Leukosis.J Virol. 2023 Jan 31;97(1):e0154222. doi: 10.1128/jvi.01542-22. Epub 2022 Dec 19. J Virol. 2023. PMID: 36533951 Free PMC article.
-
Simultaneous evaluation of diagnostic marker utility for enzootic bovine leukosis.BMC Vet Res. 2019 Nov 9;15(1):406. doi: 10.1186/s12917-019-2158-4. BMC Vet Res. 2019. PMID: 31706301 Free PMC article.
-
A rapid and simple clonality assay for bovine leukemia virus-infected cells by amplified fragment length polymorphism (AFLP) analysis.Microbiol Spectr. 2025 Jan 7;13(1):e0171424. doi: 10.1128/spectrum.01714-24. Epub 2024 Nov 21. Microbiol Spectr. 2025. PMID: 39570050 Free PMC article.
-
Emphasis on cell turnover in two hosts infected by bovine leukemia virus: a rationale for host susceptibility to disease.Vet Immunol Immunopathol. 2008 Sep 15;125(1-2):1-7. doi: 10.1016/j.vetimm.2008.04.007. Epub 2008 Apr 16. Vet Immunol Immunopathol. 2008. PMID: 18513803 Review.
-
Bovine Leukaemia Virus: Current Epidemiological Circumstance and Future Prospective.Viruses. 2021 Oct 27;13(11):2167. doi: 10.3390/v13112167. Viruses. 2021. PMID: 34834973 Free PMC article. Review.
Cited by
-
Identification of bovine leukemia virus in raw milk samples in North-West of Iran.Vet Res Forum. 2021 Spring;12(2):223-227. doi: 10.30466/vrf.2019.102686.2446. Epub 2021 Jun 15. Vet Res Forum. 2021. PMID: 34345390 Free PMC article.
-
A clinical case of enzootic bovine leukosis in a Holstein cow with minor clonality of B-cell in the peripheral blood.J Vet Med Sci. 2024 Dec 1;86(12):1289-1293. doi: 10.1292/jvms.24-0298. Epub 2024 Nov 4. J Vet Med Sci. 2024. PMID: 39496454 Free PMC article.
-
Enzootic bovine leukosis in a 21-month-old Japanese Black cow with high susceptibility.J Vet Diagn Invest. 2022 Jul;34(4):733-737. doi: 10.1177/10406387221102123. Epub 2022 Jun 10. J Vet Diagn Invest. 2022. PMID: 35686385 Free PMC article.
-
Time course changes in peripheral B-cell clonality in a Japanese Black bull with enzootic bovine leukosis.J Vet Med Sci. 2022 Nov 7;84(11):1495-1498. doi: 10.1292/jvms.22-0314. Epub 2022 Sep 29. J Vet Med Sci. 2022. PMID: 36171110 Free PMC article.
-
Clone Dynamics and Its Application for the Diagnosis of Enzootic Bovine Leukosis.J Virol. 2023 Jan 31;97(1):e0154222. doi: 10.1128/jvi.01542-22. Epub 2022 Dec 19. J Virol. 2023. PMID: 36533951 Free PMC article.
References
-
- Gillet N, Florins A, Boxus M, Burteau C, Nigro A, Vandermeers F, Balon H, Bouzar AB, Defoiche J, Burny A, Reichert M, Kettmann R, Willems L. 2007. Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology 4:18. doi:10.1186/1742-4690-4-18. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

