Role of glutamine and interlinked asparagine metabolism in vessel formation
- PMID: 28659375
- PMCID: PMC5556263
- DOI: 10.15252/embj.201695518
Role of glutamine and interlinked asparagine metabolism in vessel formation
Abstract
Endothelial cell (EC) metabolism is emerging as a regulator of angiogenesis, but the precise role of glutamine metabolism in ECs is unknown. Here, we show that depriving ECs of glutamine or inhibiting glutaminase 1 (GLS1) caused vessel sprouting defects due to impaired proliferation and migration, and reduced pathological ocular angiogenesis. Inhibition of glutamine metabolism in ECs did not cause energy distress, but impaired tricarboxylic acid (TCA) cycle anaplerosis, macromolecule production, and redox homeostasis. Only the combination of TCA cycle replenishment plus asparagine supplementation restored the metabolic aberrations and proliferation defect caused by glutamine deprivation. Mechanistically, glutamine provided nitrogen for asparagine synthesis to sustain cellular homeostasis. While ECs can take up asparagine, silencing asparagine synthetase (ASNS, which converts glutamine-derived nitrogen and aspartate to asparagine) impaired EC sprouting even in the presence of glutamine and asparagine. Asparagine further proved crucial in glutamine-deprived ECs to restore protein synthesis, suppress ER stress, and reactivate mTOR signaling. These findings reveal a novel link between endothelial glutamine and asparagine metabolism in vessel sprouting.
Keywords: angiogenesis; asparagine; endothelial cell; glutamine; metabolism.
© 2017 The Authors.
Figures

- A
Amino acid concentration in standard M199 containing 20% FBS at the starting point (T0) of the consumption/excretion experiment (Fig 1A).
- B
mRNA levels of GLS1 and GLS2 in ECs determined by qRT–PCR. cDNA from GLS2 overexpressing (GLS2‐OE) ECs was used as a positive control for the specificity of the GLS2 primer/probe set.
- C
GLS1 mRNA level in control and GLS1KD ECs.
- D
Representative Western blot for GLS1 in control and GLS1KD ECs with β‐actin as a loading control.
- E, F
[3H]‐Thymidine incorporation into DNA in control and CB‐839‐treated ECs upon GLS2 overexpression (E) or glutamate supplementation (F).
- G
Representative Western blot and detection of caspase‐3 (apoptosis marker) in ECs treated with the indicated concentrations of CB‐839. α‐Tubulin was used as a loading control. Note that none of the concentrations of CB‐839 induced activation of caspase‐3, as can be judged from the lack of the typical 17 kDa cleavage product.
- H
Representative images of TUNEL (red)/Hoechst (blue)‐stained ECs treated with the indicated concentrations of CB‐839. Doxorubicin (1 μg/ml) was used as an apoptosis inducer and served as a positive control.
- I
mRNA level of GLS1 in mouse ECs obtained from livers of GLS1ECKO mice and their wild‐type littermates (n = 3).

- A
Quantification of amino acid consumption or secretion rate (values below zero indicate consumption, values above zero indicate secretion).
- B–E
Representative images of EC spheroids sprouting under control (B) and glutamine‐free conditions (C), and corresponding quantification of total sprout length (D) and number of sprouts per spheroid (E).
- F
[3H]‐Thymidine incorporation in DNA in ECs at different doses of extracellular glutamine.
- G
Percentage of wound closure in control and glutamine‐deprived ECs in monolayer scratch migration assays.
- H, I
Quantification of glutamine uptake (H) and glutamine oxidation (I) in quiescent (Q) versus proliferating (P) ECs.
- J–M
Representative images of control (J) and GLS1KD (K) EC spheroids, and quantification of total sprout length (L) and number of sprouts per spheroid (M).
- N
[3H]‐Thymidine incorporation in DNA in control and GLS1KD ECs.
- O
Percentage of wound closure in control and GLS1KD ECs in scratch migration assays.
- P
[3H]‐Thymidine incorporation into DNA in ECs treated with increasing concentrations of the GLS1 inhibitor CB‐839.
- Q
Annexin V+ ECs (relative to control) after treatment with different concentrations of CB‐839.
- R
Percent cell death (measured by LDH release) in ECs treated with different concentrations of CB‐839.
- S
[3H]‐Thymidine incorporation in DNA in mouse liver ECs isolated from GLS1ECKO mice and their corresponding wild‐type littermates (n = 3 for both genotypes).
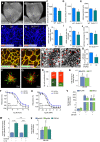
- A, B
Representative pictures from isolectin‐B4 (IB4)‐stained retinal vascular plexus obtained from wild‐type (A) or GLS1ECKO (B) mice at P5.
- C–E
Quantification of branch points at the front (C) or rear (D), and radial expansion (E) of the retinal vascular plexus in wild‐type and GLS1ECKO animals (n = 9).
- F, G
Representative pictures of IB4/phospho‐histone 3 (phH3) double‐stained wild‐type (F) and GLS1ECKO (G) retinal vasculature at P5.
- H
Quantification of phH3+ ECs in the retinal vascular plexus of wild‐type and GLS1ECKO mice (n = 6).
- I
Quantification of distal sprouts with filopodia in the retinal vasculature of wild‐type and GLS1ECKO mice at P5 (n = 7).
- J–L
Quantification of pericyte coverage (NG2+/IB4+ area in % of wild‐type) (n = 6) (J), and corresponding representative pictures of IB4/NG2 (pericyte marker) double staining of P5 wild‐type (K) and GLS1ECKO (L) retinal vascular plexus.
- M–O
Representative images of retinal flat mounts of retinopathy of prematurity (ROP) mice treated with vehicle (M) or CB‐839 (N) (red arrows indicate vascular tufts) and corresponding quantification of vascular tuft area (O) (n = 8).
- P–R
Representative pictures of mosaic spheroids consisting of a 1:1 mixture of mCherry+‐control (red) and GFP+‐control (green) ECs (P) or a 1:1 mixture of mCherry+‐control (red) and GFP+‐GLS1KD (green) ECs (Q) and quantification (per spheroid) of percentages of red versus green ECs occupying the tip cell position (R) (n = 3).
- S
Fold changes in mRNA level of eNOS and endothelin‐1 in CB‐839‐treated ECs (n = 4).
- T, U
Relaxation of aortic rings pretreated with vehicle or CB‐839 in response to acetylcholine (T) and in response to the nitric oxide donor sodium nitroprusside (U) (n = 8).
- V
Fold changes in mRNA levels of VCAM and E‐selectin in CB‐839‐treated ECs under IL‐1β stimulation (n = 4).
- W
Quantification of number of leukocytes adhering to a vehicle‐ or CB‐839‐treated EC monolayer under IL‐1β stimulation (n = 4).
- X
Fold changes in mRNA level of arterial (ephrin B2), venous (ephrin B4), and lymphatic (Prox1) markers in CB‐839‐treated ECs (n = 4).
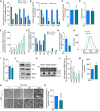
- A
Gas chromatography–mass spectrometry (GC‐MS)‐based analysis of total contribution of [U‐13C]‐glutamine and [U‐13C]‐glucose to tricarboxylic acid (TCA) intermediates in ECs. [U‐13C]‐Glutamine and [U‐13C]‐glucose were supplemented to the culture medium at 2 and 5.5 mM final concentration, respectively. All labelings were performed under steady‐state conditions.
- B
Quantification of intracellular TCA metabolites in control and glutamine‐deprived (Gln free) ECs.
- C
Intracellular ATP levels measured by liquid chromatography–mass spectrometry (LC‐MS) in control and glutamine‐depleted ECs.
- D
Energy charge measurement in control and glutamine‐deprived ECs.
- E
Intracellular levels of the non‐essential amino acids glutamate, aspartate, asparagine, alanine, glycine, and serine in control and glutamine‐deprived ECs. Level in control cells is indicated by the dashed line.
- F
Total contribution of [U‐13C]‐glutamine carbon and [15N2]‐glutamine nitrogen to non‐essential amino acids in ECs.
- G
Measurement of protein synthesis rate ([3H]‐tyrosine incorporation) in control and glutamine‐deprived ECs.
- H, I
Representative cell size distribution graph (H) and corresponding quantification of cell sizes (I) measured by forward scatter value of flow cytometry in control and glutamine‐deprived ECs.
- J
mTOR signaling activity in control and glutamine‐deprived ECs, revealed by immunoblotting for phosphorylated ribosomal protein S6 (p‐S6) and by 4EBP1 mobility shift. β‐Actin was used as loading control. Representative blot of three independent experiments is shown.
- K
Measurement of ER stress in control and glutamine‐deprived ECs, determined by accumulation of ATF4 and Chop protein. β‐actin was used as a loading control. Results from three independent experiments are shown. Asterisk indicates a non‐specific band.
- L
mRNA levels of ER stress marker genes in glutamine‐deprived versus control ECs determined by qRT–PCR.
- M
Intracellular reactive oxygen species (ROS) levels in control and glutamine‐deprived ECs measured by CM‐DCF staining.
- N
Representative pictures of control and glutamine‐deprived ECs at 2 h after treatment with increasing concentrations of H2O2. In glutamine starvation (but not control) conditions, treatment with 250 μM H2O2 already resulted in cell death, revealed by loss of normal cell morphology.
- O
Total glutathione content in control and glutamine‐deprived ECs.
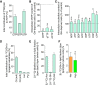
Total contribution of [15N2]‐glutamine to ribonucleotide triphosphates (rNTPs) in ECs.
Quantification of deoxynucleotides (dNTP) in glutamine‐deprived ECs; the level in control ECs is indicated by the dashed line.
Quantification of intracellular ribonucleotide content in glutamine‐deprived ECs; the level in control ECs is indicated by the dashed line.
Quantification of total contribution of [U‐13C]‐glutamine to m + 4 citrate (Cit(m4)), m + 5 citrate (Cit(m5)), pyruvate (Pyr), and lactate (Lac) in ECs.
Quantification of percentage of [U‐14C]‐acetate (Ace), [U‐14C]‐glucose (Glc), and [U‐14C]‐glutamine (Gln) used for lipid production in ECs under normal culture conditions.
Quantification of percentage of [U‐14C]‐glutamine (Gln) used for lipid production in ECs cultured in normoxia (Nor) or hypoxia (Hyp, 0.5% O2).

- A
Quantification of cell numbers in glutamine‐starved ECs during culture with or without nucleotide supplementation. dNTPs and NSX refer to deoxyribonucleotide triphosphates and nucleoside mixture, respectively.
- B–E
[3H]‐Thymidine incorporation into DNA in glutamine‐starved ECs with or without supplementation with exogenous carbon sources (B), NEAAs (C), or the antioxidant NAC (D) or GSH‐EE (E). Pyr, pyruvate; Suc, mono‐methyl hydrogen succinate; OAA, oxaloacetate; NEAA: pool of glycine, alanine, asparagine, aspartate, glutamate, proline, and serine. NAC, N‐acetylcysteine. GSH‐EE, glutathione reduced ethyl ester.
- F
[3H]‐Thymidine incorporation into DNA in glutamine‐starved ECs with our without asparagine supplementation combined with exogenous carbon sources.
- G
ASNS mRNA levels in control ECs, ECs grown under hypoxia and thapsigargin (thap.; ER stress inducer)‐treated ECs.
- H
Representative Western blot for ASNS in control and ASNSKD ECs with β‐actin as a loading control. Red asterisk indicates non‐specific band.
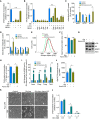
- A
[3H]‐Thymidine incorporation in DNA in glutamine‐starved ECs with and without supplementation of exogenous cell‐permeable dimethyl α‐ketoglutarate (hereafter referred to as α‐KG) or a non‐essential amino acid mixture (NEAA).
- B
[3H]‐Thymidine incorporation in DNA in glutamine‐starved ECs with and without single or combined supplementation of exogenous α‐KG and individual NEAAs.
- C
Quantification of intracellular deoxynucleotide (dNTP) levels in control and glutamine‐free (without or with supplementation of asparagine and α‐KG) conditions.
- D
Quantification of intracellular TCA metabolites in control or glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG.
- E, F
Representative graph of cell size distribution (E) determined by flow cytometry and corresponding quantification of cell size (F) of control or glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG. Of note, the first two bars in this graph are the same as in Fig 3I. The data in the two panels originate from the same set of experiments, only in panel (F) an additional condition is displayed.
- G
mTOR activation, revealed by immunoblotting for phosphorylated ribosomal protein S6 (p‐S6) and by 4EBP1 mobility shift, in control or glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG. β‐Actin was used as loading control. Immunoblots shown are representative of three independent experiments.
- H
Protein synthesis ([3H]‐tyrosine incorporation assay) in control and glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG.
- I
mRNA levels of ER stress markers in control or glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG.
- J
Intracellular reactive oxygen species (ROS) levels (CM‐DCF staining) in control and glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG.
- K
Representative pictures of control and glutamine‐deprived ECs with and without combined supplementation of asparagine and α‐KG at baseline and at 2 h after treatment with H2O2.
- L
[3H]‐Thymidine incorporation into DNA in ECs grown at different concentrations of glutamine, with or without supplementation of asparagine.

- A
mRNA level of ASNS in control and ASNSKD ECs.
- B–E
Quantification of number of sprouts (B) and total sprout length (C) in control and ASNSKD EC spheroids and corresponding representative images (D, E).
- F
[3H]‐Thymidine incorporation into DNA in control and ASNSKD ECs with or without asparagine supplementation.
- G
Protein synthesis ([3H]‐tyrosine incorporation assay) in control and glutamine‐deprived ECs with and without supplementation of asparagine. Of note, the first two bars in this graph are the same as in Fig 4H. The data in the two panels originate from the same set of experiments, only in panel (G) another third condition is displayed.
- H
mRNA and protein levels of ER stress markers in control and glutamine‐deprived ECs with and without supplementation of asparagine.
- I
Representative immunoblots of mTOR activation, revealed by immunoblotting for phosphorylated ribosomal protein S6 (p‐S6) and 4EBP1 mobility shift, in control and glutamine‐deprived ECs with and without single or combined supplementation of asparagine and α‐KG. Of note, the first three lanes of the β‐actin loading control are the same as the β‐actin loading controls in panel (H) because the Chop immunoblotting was done in the same experiment but was found to fit more logically in panel (H).
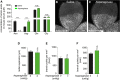
- A
Quantification of asparagine, aspartate, glutamine, and glutamate levels in serum of P4 mouse pups treated with saline or 2 U/g/day asparaginase.
- B, C
Representative pictures of the isolectin‐B4‐stained retinal microvasculature in vehicle‐ (B) and asparaginase‐treated (C) P4 pups. Scale bars are 250 μm.
- D
Radial outgrowth of the vascular plexus in vehicle‐ and asparaginase‐treated pups.
- E, F
Quantification of the number of branchpoints in the front (E) and the rear (F) part of the microvascular plexus in vehicle‐ and asparaginase‐treated pups.
Comment in
-
New Q(ues) to keep blood vessels growing.EMBO J. 2017 Aug 15;36(16):2315-2317. doi: 10.15252/embj.201797764. Epub 2017 Aug 4. EMBO J. 2017. PMID: 28778957 Free PMC article.
References
-
- Ahlman B, Andersson K, Leijonmarck CE, Ljungqvist O, Hedenborg L, Wernerman J (1994) Short‐term starvation alters the free amino acid content of the human intestinal mucosa. Clin Sci (Lond) 86: 653–662 - PubMed
-
- Armstrong MD, Stave U (1973) A study of plasma free amino acid levels. II. Normal values for children and adults. Metabolism 22: 561–569 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

