Glutamine fuels proliferation but not migration of endothelial cells
- PMID: 28659379
- PMCID: PMC5556269
- DOI: 10.15252/embj.201796436
Glutamine fuels proliferation but not migration of endothelial cells
Abstract
Endothelial metabolism is a key regulator of angiogenesis. Glutamine metabolism in endothelial cells (ECs) has been poorly studied. We used genetic modifications and 13C tracing approaches to define glutamine metabolism in these cells. Glutamine supplies the majority of carbons in the tricyclic acid (TCA) cycle of ECs and contributes to lipid biosynthesis via reductive carboxylation. EC-specific deletion in mice of glutaminase, the initial enzyme in glutamine catabolism, markedly blunts angiogenesis. In cell culture, glutamine deprivation or inhibition of glutaminase prevents EC proliferation, but does not prevent cell migration, which relies instead on aerobic glycolysis. Without glutamine catabolism, there is near complete loss of TCA intermediates, with no compensation from glucose-derived anaplerosis. Mechanistically, addition of exogenous alpha-ketoglutarate replenishes TCA intermediates and rescues cellular growth, but simultaneously unveils a requirement for Rac1-dependent macropinocytosis to provide non-essential amino acids, including asparagine. Together, these data outline the dependence of ECs on glutamine for cataplerotic processes; the need for glutamine as a nitrogen source for generation of biomass; and the distinct roles of glucose and glutamine in EC biology.
Keywords: endothelium; glutamine; macropinocytosis; migration.
© 2017 The Authors.
Figures

Schematic of labeling scheme, using either [U‐13C] glucose or [U‐13C] glutamine. For simplicity, labeling of only the first round in TCA cycle is shown.
HUVECs were cultured for 24 h in the presence of tracer (either [U‐13C] glucose (Glc) and 12C glutamine (Gln) or 12C Glc and [U‐13C] Gln), followed by mass spectrometry of the indicated metabolites. The color codes indicate the % of each metabolite that is heavy isotope‐labeled by the indicated number of carbons. M+n: a metabolite with n carbon atoms labeled with 13C.
Total percentage of carbons, for each TCA metabolite, that is heavy isotope‐labeled in the presence of [U‐13C] Glc or [U‐13C] Gln. Approximately 70% of carbons in the TCA cycle derive from glutamine, while less than 20% of carbons derive from glucose.

Glutamine is routed to lipids via reductive carboxylation. Left top: Schematic depicting three approaches to estimate reductive glutamine metabolism with [U‐13C] glutamine. (1) Ratio of M+5 citrate to M+5 aKG indicates the portion of citrate that is derived from aKG (12.5%). (2) Ratio of M+3 to M+4 4‐carbon members of TCA cycle indicates the portion that are derived from oxidation versus reduction in aKG (15.4% for aspartate, 9.9% for fumarate, and 13.8% for malate). (3) Ratio of labeled FA carbons from [U‐13C] Glc versus [U‐13C] Gln reflects the portion of citrate that is derived from aKG versus condensation with glucose‐derived Ac‐CoA.
Schematic depicting estimation of malic enzyme (ME) flux from [U‐13C] Gln. The ratio of M+6 to M+4 citrate suggests ME activity.
Labeling of non‐essential amino acids (proline, aspartate, asparagine, and glutamate) from [U‐13C] Gln.
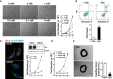
EC proliferation is dependent on glutamine. Growth curves (right) and phase contrast images (40×, left) of HUVECs after 4 days of culture in medium with the indicated concentrations of glutamine. Scale bar = 100 μm.
Glutamine depletion induces apoptotic cell death, assessed by Annexin V and PI staining 4 days after glutamine (Gln) depletion.
GLS is located in the mitochondria, and knockdown of GLS impairs EC proliferation. Immunofluorescence (400×, left) for GLS (red), Tom20 (green) and DAPI (blue), and immunoblotting (top right) for GLS and GAPDH at day 4 of culture. Growth curve (bottom right) of HUVECs transfected with siRNA against GLS and control (CTL) in glutamine‐containing medium. Scale bar = 10 μm.
Growth curve of HUVECs treated with the indicated doses of selective GLS inhibitor BPTES. For all proliferation assay, cells are counted by TC20™ Automated Cell Counter.
Glutamine depletion impairs capillary sprouting from mouse aortic explants. At day 3 of aortic ring explant incubation, each explant was photographed and the area of capillary outgrowth was quantified using ImageJ. Scale bar = 1 mm.
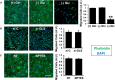
- A–C
Migration of HUVECs was quantified in the presence or absence of glutamine (Gln) or glucose (Glc) (A), or treated with si‐control (C) versus si‐GLS (B), or treated with BPTES versus no treatment (NT) (C). The same number of cells was seeded into the upper chamber of transwell plates and allowed to migrate for 12 h. Cells that migrated across the transwell membrane were visualized by staining with phalloidin (green) and DAPI (blue). Glc: glucose; Gln: glutamine. Scale bar = 100 μm. Data shown as mean ± SD, n = 6. (+)Gln versus (−)Glc, **P < 0.01. P‐values were calculated by using two‐tailed Student's t‐test.

- A–G
Staining of WT versus GLSΔEC mouse retina at postnatal day (P)7. Tamoxifen was injected for three consecutive days from P1 to P3, and retinal vasculature was analyzed at P7. (A) The entire retinal vasculature is visualized by IB4 staining. Scale bar = 1 mm. (B) Arteriovenous (a/v) patterning was analyzed by IB4 and SMA staining (C–E). Vascular density (angiogenic front: C and rear plexus: E), number of branching points, and number of pH3‐positive cells were analyzed by either CD31, IB4, pH3, or ERG staining. Number of tip cells (F) and filopodia (G) are visualized via IB4 staining under high‐power magnification. Quantifications are at bottom of figure and represent data from three independent experiments (mean ± SD, n = 7–9, WT versus GLSΔEC, **P < 0.01). Magnification of objective lens (A: 2×, B: 5×, C and E: 10×, D and F: 20×, G: 63×). Scale bars = 50 μm. White arrowheads indicate pH3‐positive proliferating ECs, red triangles indicate tip cells, yellow dots indicate filopodia, red dotted lines indicate body of tip cells, and blue dotted lines indicate the boundary of tip cell and stalk cell. P‐values were calculated by using two‐tailed Student's t‐test.
- H
Capillary sprouting of aortic explants from WT versus GLSΔEC mouse. At day 3 of aortic ring explant incubation, each explant was photographed and the area of capillary outgrowth was quantified using ImageJ. Data are represented as mean ± SD, n > 4 segments per aorta from three mice each. WT versus GLSΔEC, *P < 0.05. P‐values were calculated by using two‐tailed Student's t‐test.

Relative abundance of TCA cycle and glycolysis metabolites. HUVECs were cultured with (+ Gln) or without (− Gln) glutamine (upper panel) or transfected with control or GLS siRNA (lower panel) and intracellular TCA cycle (left panel) and glycolysis (right panel) intermediates were quantified by mass spectrometry after 24 h of incubation.
Absence of compensation by pyruvate dehydrogenase (PDH) or pyruvate carboxylase (PC). Left: Schematic depicting approach to estimate relative PC and PDH flux from [U‐13C] Glc. Right top: Relative PDH activity in si‐control (C) versus si‐Gls conditions, estimated by M+2 labeled of TCA intermediates. Right bottom: Relative PC activity identified by comparing the M+3/M+2 ratio of succinate to that of fumarate. Rightmost bottom: M+5 citrate as an additional measure of relative PC activity.

aKG supplementation restores intracellular TCA cycle intermediates in glutamine‐depleted conditions. Cell‐permeable aKG (7 mM) was added to glutamine‐depleted media, and the abundance of TCA cycle intermediates was measured by mass spectrometry. Values are indicated as relative to (+)Gln condition (dotted line).
aKG supplementation rescues proliferation in glutamine‐depleted condition. Growth curve and phase contrast images (40×) of HUVECs during 4 days of culture in (+)Gln, (−)Gln, and (−)Gln (+)αKG condition. Scale bar = 100 μm.
aKG supplementation similarly rescues proliferation in si‐Gls condition.
aKG supplementation rescues viability in glutamine‐depleted condition. Cell death was assessed by Annexin V and PI staining after 4 days of culture in the indicated condition.

- A
Macropinocytosis is increased in the absence of glutamine. HUVECs were cultured in the presence and absence of glutamine with or without aKG or Amil (amiloride), and macropinocytosis was measured by Texas Red‐Dextran uptake assay. Representative images are shown on the left, and quantification on the right. NT: no treatment. Scale bar = 10 μm.
- B
Addition of 3% BSA promotes cell growth in glutamine‐depleted and aKG‐supplemented condition. HUVECs were cultured in the glutamine‐depleted and aKG‐supplemented medium with or without 3% BSA for 4 days.
- C, D
Macropinocytosis is necessary for EC survival and nitrogen source in glutamine‐depleted and aKG‐supplemented condition. (C) Left: HUVECs die upon amiloride (+ Amil) treatment, and viability is rescued by supplementation of non‐essential amino acids (NEAA, 0.2 mM). Right: Growth curves of HUVECs under the same three conditions. Scale bar = 100 μm. (D) Growth curves of HUVECs treated with si‐RAC1 versus si‐Control and si‐RAC1 rescued with NEAA, as in (C).
- E
Schematic of compartmental use of glucose for ATP generation versus glutamine for generation of biomass (see text for details).
Comment in
-
New Q(ues) to keep blood vessels growing.EMBO J. 2017 Aug 15;36(16):2315-2317. doi: 10.15252/embj.201797764. Epub 2017 Aug 4. EMBO J. 2017. PMID: 28778957 Free PMC article.
-
Role of Cellular Metabolism in Pulmonary Diseases.Am J Respir Cell Mol Biol. 2018 Jul;59(1):127-129. doi: 10.1165/rcmb.2018-0103RO. Am J Respir Cell Mol Biol. 2018. PMID: 29634283 Free PMC article. No abstract available.
References
-
- Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, Jones DT, Vojnovic B, Hodivala‐Dilke K (2012) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7: 89–104 - PubMed
-
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I et al (2013) Role of PFKFB3‐driven glycolysis in vessel sprouting. Cell 154: 651–663 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

