Early antiretroviral therapy and potent second-line drugs could decrease HIV incidence of drug resistance
- PMID: 28659449
- PMCID: PMC5489726
- DOI: 10.1098/rspb.2017.0525
Early antiretroviral therapy and potent second-line drugs could decrease HIV incidence of drug resistance
Abstract
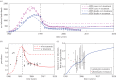
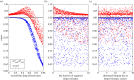
Early initiation of antiretroviral therapy (ART) reduces the risk of drug-sensitive HIV transmission but may increase the transmission of drug-resistant HIV. We used a mathematical model to estimate the long-term population-level benefits of ART and determine the scenarios under which earlier ART (treatment at 1 year post-infection, on average) could decrease simultaneously both total and drug-resistant HIV incidence (new infections). We constructed an infection-age-structured mathematical model that tracked the transmission rates over the course of infection and modelled the patients' life expectancy as a function of ART initiation timing. We fitted this model to the annual AIDS incidence and death data directly, and to resistance data and demographic data indirectly among men who have sex with men (MSM) in San Francisco. Using counterfactual scenarios, we assessed the impact on total and drug-resistant HIV incidence of ART initiation timing, frequency of acquired drug resistance, and second-line drug effectiveness (defined as the combination of resistance monitoring, biomedical drug efficacy and adherence). Earlier ART initiation could decrease the number of both total and drug-resistant HIV incidence when second-line drug effectiveness is sufficiently high (greater than 80%), but increase the proportion of new infections that are drug resistant. Thus, resistance may paradoxically appear to be increasing while actually decreasing.
Keywords: acquired drugresistance; early antiretroviral therapy initiation; mathematical model; second-line drug effectiveness; transmission of drug-resistant HIV.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD, the HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338, 853–860. (10.1056/NEJM199803263381301) - DOI - PubMed
-
- Detels R, Muñoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP, the Multicenter AIDS Cohort Study Investigators. 1998. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. J. Am. Med. Assoc. 280, 1497–1503. (10.1001/jama.280.17.1497) - DOI - PubMed
-
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Nachega JB, Dybul M, Hogg RS. 2011. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann. Intern. Med. 155, 209–216. (10.7326/0003-4819-155-4-201108160-00358) - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

