Cellular uptake of proMMP-2:TIMP-2 complexes by the endocytic receptor megalin/LRP-2
- PMID: 28659595
- PMCID: PMC5489529
- DOI: 10.1038/s41598-017-04648-y
Cellular uptake of proMMP-2:TIMP-2 complexes by the endocytic receptor megalin/LRP-2
Abstract
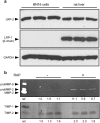
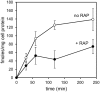
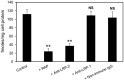
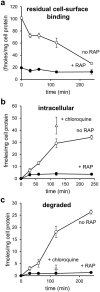
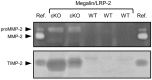
Matrix metalloproteinases (MMPs) are regulated at multiple transcriptional and post-transcriptional levels, among which receptor-mediated endocytic clearance. We previously showed that low-density lipoprotein receptor-related protein-1 (LRP-1) mediates the clearance of a complex between the zymogen form of MMP-2 (proMMP-2) and tissue inhibitor of metalloproteinases, TIMP-2, in HT1080 human fibrosarcoma cells. Here we show that, in BN16 rat yolk sac cells, proMMP-2:TIMP-2 complex is endocytosed through a distinct LRP member, megalin/LRP-2. Addition of receptor-associated protein (RAP), a natural LRP antagonist, caused accumulation of endogenous proMMP-2 and TIMP-2 in conditioned media. Incubation with RAP also inhibited membrane binding and cellular uptake of exogenous iodinated proMMP-2:TIMP-2. Moreover, antibodies against megalin/LRP-2, but not against LRP-1, inhibited binding of proMMP-2:TIMP-2 to BN16 cell surface. BIAcore analysis confirmed direct interaction between the complex and megalin/LRP-2. Conditional renal invalidation of megalin/LRP-2 in mice resulted in accumulation of proMMP-2 and TIMP-2 in their urine, highlighting the physiological relevance of the binding. We conclude that megalin/LRP-2 can efficiently mediate cell-surface binding and endocytosis of proMMP-2:TIMP-2 complex. Therefore megalin/LRP-2 can be considered as a new actor in regulation of MMP-2 activity, an enzyme crucially involved in many pathological processes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2.TIMP-2 complex through a thrombospondin-independent mechanism.J Biol Chem. 2004 Dec 24;279(52):54944-51. doi: 10.1074/jbc.M406792200. Epub 2004 Oct 15. J Biol Chem. 2004. PMID: 15489233
-
Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase A (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP).Biochem J. 2003 Jun 15;372(Pt 3):799-809. doi: 10.1042/BJ20021573. Biochem J. 2003. PMID: 12630911 Free PMC article.
-
Peptide from the C-terminal domain of tissue inhibitor of matrix metalloproteinases-2 (TIMP-2) inhibits membrane activation of matrix metalloproteinase-2 (MMP-2).Matrix Biol. 2011 Sep;30(7-8):404-12. doi: 10.1016/j.matbio.2011.07.001. Epub 2011 Aug 4. Matrix Biol. 2011. PMID: 21839835 Free PMC article.
-
Interaction between tissue inhibitor of metalloproteinases-2 and progelatinase A: immunoreactivity analyses.Biochem J. 1996 Feb 1;313 ( Pt 3)(Pt 3):827-33. doi: 10.1042/bj3130827. Biochem J. 1996. PMID: 8611162 Free PMC article.
-
Cell surface activation of progelatinase A (proMMP-2) and cell migration.Cell Res. 1998 Sep;8(3):179-86. doi: 10.1038/cr.1998.18. Cell Res. 1998. PMID: 9791731 Review.
Cited by
-
Vacuolar H+-ATPase and Megalin-Mediated Prorenin Uptake: Focus on Elements Beyond the (Pro)Renin Receptor.J Cell Physiol. 2025 Jan;240(1):e31518. doi: 10.1002/jcp.31518. J Cell Physiol. 2025. PMID: 39745029 Free PMC article.
-
MMP2 and TLRs modulate immune responses in the tumor microenvironment.JCI Insight. 2021 Jun 22;6(12):e144913. doi: 10.1172/jci.insight.144913. JCI Insight. 2021. PMID: 34032639 Free PMC article.
-
Reflections on the evolution of the vertebrate tissue inhibitors of metalloproteinases.FASEB J. 2019 Jan;33(1):71-87. doi: 10.1096/fj.201801262R. Epub 2018 Aug 20. FASEB J. 2019. PMID: 30125136 Free PMC article. Review.
-
Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications.Physiol Rev. 2022 Oct 1;102(4):1625-1667. doi: 10.1152/physrev.00014.2021. Epub 2022 Apr 4. Physiol Rev. 2022. PMID: 35378997 Free PMC article. Review.
-
Targeting the MMP-14/MMP-2/integrin αvβ3 axis with multispecific N-TIMP2-based antagonists for cancer therapy.J Biol Chem. 2018 Aug 24;293(34):13310-13326. doi: 10.1074/jbc.RA118.004406. Epub 2018 Jul 9. J Biol Chem. 2018. PMID: 29986882 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

